null
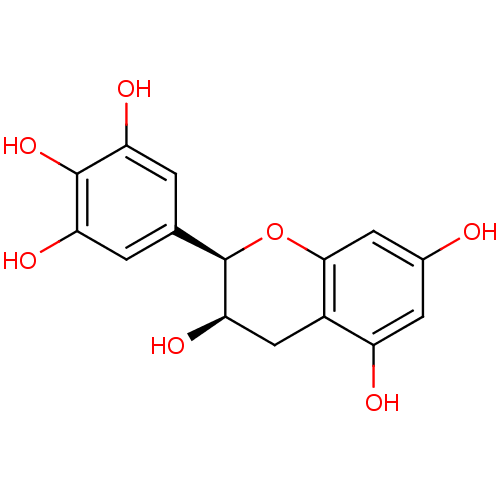
SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1
InChI Key InChIKey=XMOCLSLCDHWDHP-IUODEOHRSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50187665
Found 19 hits for monomerid = 50187665
TargetEnoyl-acyl-carrier protein reductase(Plasmodium falciparum)
National Institute of Immunology
Curated by ChEMBL
National Institute of Immunology
Curated by ChEMBL
Affinity DataKi: 17.6nMAssay Description:Inhibition of Plasmodium falciparum ENR in presence of triclosanMore data for this Ligand-Target Pair
TargetEnoyl-acyl-carrier protein reductase(Plasmodium falciparum)
National Institute of Immunology
Curated by ChEMBL
National Institute of Immunology
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of Plasmodium falciparum ENR using crotonyl-CoA substrateMore data for this Ligand-Target Pair
TargetEnoyl-acyl-carrier protein reductase(Plasmodium falciparum)
National Institute of Immunology
Curated by ChEMBL
National Institute of Immunology
Curated by ChEMBL
Affinity DataKi: 3.75E+3nMAssay Description:Inhibition of Plasmodium falciparum ENR using NADH substrateMore data for this Ligand-Target Pair
Affinity DataKi: 3.57E+4nMAssay Description:Displacement of [3H]-CP55940 from CB1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of NLWAAQRYGRELRRMSD-K(FITC)-FVD from Bcl-2 (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.61E+5nMAssay Description:Displacement of [3H]-CP55940 from CB2 receptor (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of aldose reductase in rat lens homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of CMG2 (40 to 217) C175A and R40C double mutant (unknown origin) interaction to full length PA E733C mutant expressed in Escherichia coli...More data for this Ligand-Target Pair
TargetProteasome subunit beta type-5(Homo sapiens (Human))
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Institute of Agricultural and Food Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.20E+6nMAssay Description:Inhibition of chymotrypsin-like activity of purified human 20S proteasome assessed as decrease in AMC hydrolysis using Suc-Leu-Leu-Val-Tyr-AMC as sub...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of 6His-tagged human RD52 assessed as reduction in RD52 binding to Cy3-dT30-Cy5 ssDNA incubated for 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibition of human alpha-synuclein filament formation expressed in Escherichia coli BL21(DE3) cells incubated for 72 hrs by thioflavin S based fluor...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] FabI(Escherichia coli)
National Institute of Immunology
Curated by ChEMBL
National Institute of Immunology
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Escherichia coli ENRMore data for this Ligand-Target Pair
TargetEnoyl-acyl-carrier protein reductase(Plasmodium falciparum)
National Institute of Immunology
Curated by ChEMBL
National Institute of Immunology
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of Plasmodium falciparum ENRMore data for this Ligand-Target Pair
Target6-phosphogluconate dehydrogenase, decarboxylating(Homo sapiens (Human))
AmorePacific Corporation
Curated by ChEMBL
AmorePacific Corporation
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of 6PGDMore data for this Ligand-Target Pair
TargetGlucose-6-phosphate 1-dehydrogenase(Saccharomyces cerevisiae S288c)
AmorePacific Corporation
Curated by ChEMBL
AmorePacific Corporation
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of yeast G6PDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Escherichia coli recombinant ribonuclease H after 30 mins by FRET quenching assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Escherichia coli recombinant ribonuclease H after 30 mins by FRET quenching assayMore data for this Ligand-Target Pair