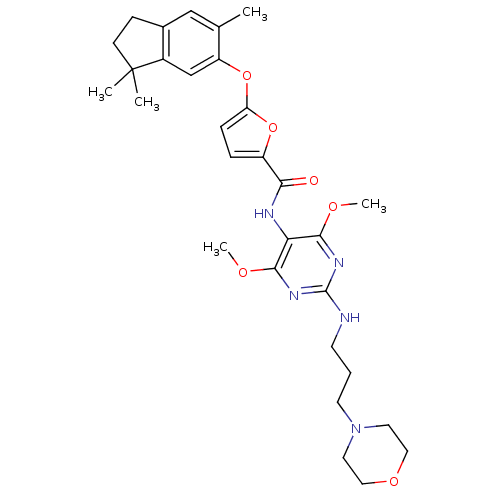
null
SMILES COc1nc(NCCCN2CCOCC2)nc(OC)c1NC(=O)c1ccc(Oc2cc3c(CCC3(C)C)cc2C)o1
InChI Key InChIKey=IGTBNUVMBDYPQG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 28 hits for monomerid = 50187673
Found 28 hits for monomerid = 50187673
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to human recombinant GnRH receptorMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Binding affinity to rat pituitary GnRH receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 583nMAssay Description:Binding affinity to human 5HT2A receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 628nMAssay Description:Binding affinity to human A3 receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 964nMAssay Description:Binding affinity to human 5HT2C receptorMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.06E+3nMAssay Description:Binding affinity to PAFMore data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.18E+3nMAssay Description:Binding affinity to human D3 receptorMore data for this Ligand-Target Pair
TargetHistamine H2 receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.22E+3nMAssay Description:Binding affinity to H2 receptorMore data for this Ligand-Target Pair
TargetSubstance-P receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.25E+3nMAssay Description:Binding affinity to human NK1 receptorMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Binding affinity to human mu opiate receptorMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.43E+3nMAssay Description:Binding affinity to human 5HT transporterMore data for this Ligand-Target Pair
TargetD(1A) dopamine receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.62E+3nMAssay Description:Binding affinity to human D1 receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.75E+3nMAssay Description:Binding affinity to human 5HT7 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Binding affinity to human DA transporterMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 1.96E+3nMAssay Description:Binding affinity to kappa opiate receptorMore data for this Ligand-Target Pair
TargetD(4) dopamine receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Binding affinity to human D4.4 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 3.68E+3nMAssay Description:Binding affinity to human M4 receptorMore data for this Ligand-Target Pair
TargetDimer of Type-1 angiotensin II receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 3.71E+3nMAssay Description:Binding affinity to human AT1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 4.12E+3nMAssay Description:Binding affinity to human M1 receptorMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 5.09E+3nMAssay Description:Binding affinity to human NE transporterMore data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataKi: 5.24E+3nMAssay Description:Binding affinity to verapamil site of N type calcium channelMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at human recombinant GnRH receptor assessed as inhibition of GnRH-stimulated inositol phosphate accumulationMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Rattus norvegicus)
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 8.80nMAssay Description:Antagonist activity at rat pitutary GnRH receptor assessed as inhibition of GnRH-stimulated inositol phosphate accumulationMore data for this Ligand-Target Pair