null
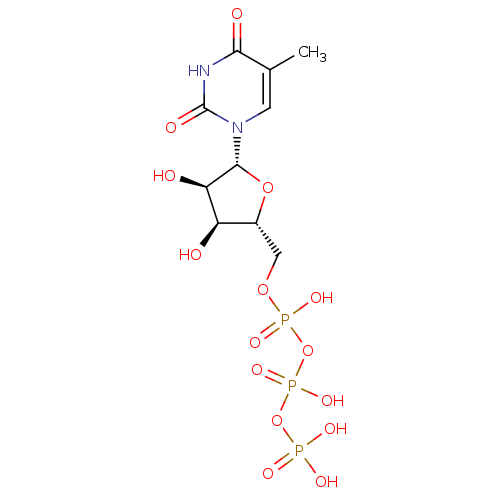
SMILES Cc1cn([C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)[nH]c1=O
InChI Key InChIKey=RZCIEJXAILMSQK-JXOAFFINSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50205415
Found 12 hits for monomerid = 50205415
Affinity DataKi: 3.70E+3nMAssay Description:Evaluated for the mixed objective Non-competitive inhibition constant Ki against TdR varied rat mitochondrial thymidine kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMAssay Description:Evaluated for the Non-competitive inhibition constant Ki against TdR varied rat cytoplasmic soluble thymidine kinaseMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus 1)
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Compound was evaluated for 50% inhibition of HIV-RT (HIV reverse transcriptase)More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus)More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Veterans Affairs Medical Center
Curated by ChEMBL
Veterans Affairs Medical Center
Curated by ChEMBL
Affinity DataKd: 2.10E+3nMAssay Description:Binding affinity to HIV1 reverse transcriptase assessed as L-3'-azido-NTP incorporation in nascent DNAMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 2(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: 480nMAssay Description:Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase CMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 6(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: 140nMAssay Description:Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase CMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 4(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: 3.90E+3nMAssay Description:Agonist activity at human recombinant P2Y4 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase CMore data for this Ligand-Target Pair
TargetReverse transcriptase protein(Human immunodeficiency virus 1)
Veterans Affairs Medical Center
Curated by ChEMBL
Veterans Affairs Medical Center
Curated by ChEMBL
Affinity DataKd: 2.90E+3nMAssay Description:Binding affinity to HIV1 reverse transcriptase M184V mutant assessed as L-3'-azido-NTP incorporation in nascent DNAMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Compound was evaluated for 50% inhibition of DHBV DNA polymerase (duck hepatitis B virus)More data for this Ligand-Target Pair
TargetDNA polymerase subunit gamma-1(Homo sapiens (Human))
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Affinity DataIC50: 8.00E+4nMAssay Description:Compound was evaluated for the inhibition of cellular DNA polymerase (gamma)More data for this Ligand-Target Pair
TargetDNA polymerase beta(Homo sapiens (Human))
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Max-Delbr�ck-Centrum f�r Molekulare Medizin
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Compound was evaluated for the inhibition of cellular DNA polymerase (beta)More data for this Ligand-Target Pair