null
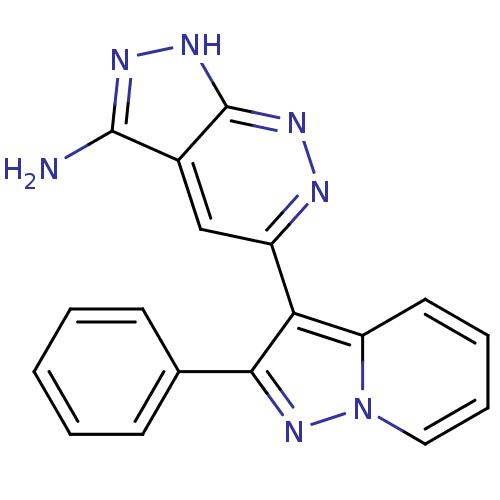
SMILES Nc1n[nH]c2nnc(cc12)-c1c(nn2ccccc12)-c1ccccc1
InChI Key InChIKey=XVECMUKVOMUNLE-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50229978
Found 9 hits for monomerid = 50229978
Affinity DataKi: 310nMAssay Description:Binding affinity to ERK2Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Inhibition of ERK1Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Inhibition of ERK2Checked by AuthorMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataKi: 310nMAssay Description:Inhibition of p38alphaChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Binding affinity to ERK1Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Binding affinity to non-phosphorylated full length N-terminal His6-tagged/SUMO-fused ERK2 (1 to 360 residues) (unknown origin) by isothermal titratio...More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+4nMAssay Description:Binding affinity to LPP-dephosphorylated recombinant wild-type ERK3 (9 to 327 residues) (unknown origin) expressed in Escherichia coli by microscale ...More data for this Ligand-Target Pair
Affinity DataKd: 850nMAssay Description:Binding affinity to ERK2 (unknown origin) by isothermal titration calorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibitory activity against human ERK2More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)