null
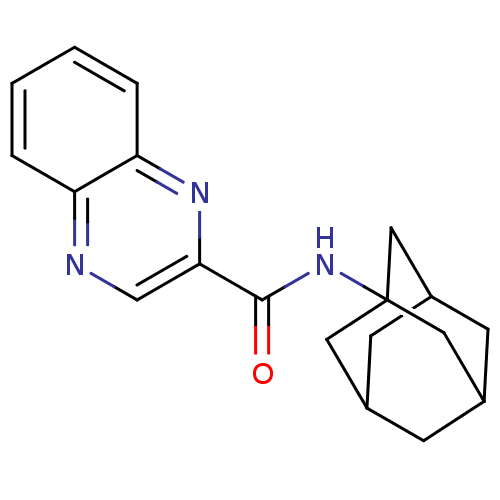
SMILES O=C(NC12CC3CC(CC(C3)C1)C2)c1cnc2ccccc2n1
InChI Key InChIKey=ZKFVOZCCAXQXBU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50231744
Found 3 hits for monomerid = 50231744
TargetMetabotropic glutamate receptor 1(RAT)
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
TargetMetabotropic glutamate receptor 1(RAT)
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
TargetMetabotropic glutamate receptor 1(RAT)
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
Johnson and Johnson Pharmaceutical Research and Development, Beerse
Curated by PDSP Ki Database
Affinity DataIC50: 5nMAssay Description:Antagonist activity at rat mGluR1 expressed in cerebellar granule cells assessed as accumulation of [3H]inositol phosphateMore data for this Ligand-Target Pair