null
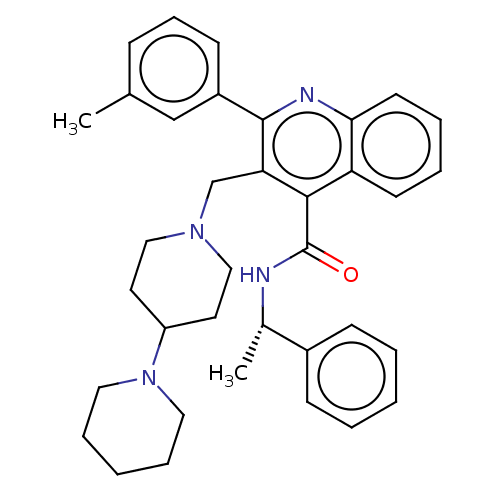
SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1cccc(C)c1)c1ccccc1
InChI Key InChIKey=ACHODVGTADPIHB-MHZLTWQESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50232811
Found 2 hits for monomerid = 50232811
TargetTransient receptor potential cation channel subfamily V member 4(Rattus norvegicus)
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Antagonist activity at rat TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins follo...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 4(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Antagonist activity at human TRPV4 expressed in HEK293 cells assessed as inhibition of GSK634775A-induced calcium influx preincubated for 10 mins fol...More data for this Ligand-Target Pair