null
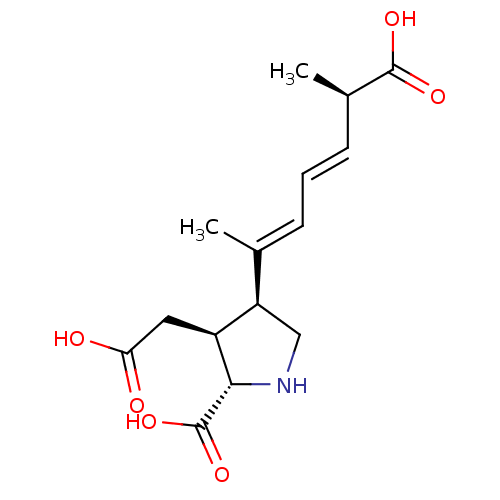
SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O
InChI Key InChIKey=VZFRNCSOCOPNDB-JIUSADRUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50252103
Found 11 hits for monomerid = 50252103
Affinity DataKi: 1.11nMAssay Description:Displacement of [3H]SYM2081 from rat recombinant iGluR5More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Universite Blaise Pascal
Curated by ChEMBL
Universite Blaise Pascal
Curated by ChEMBL
Affinity DataKi: 3.84nMAssay Description:Displacement of [3H]SYM2081 from rat recombinant iGluR7More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 2(Rattus norvegicus)
Universite Blaise Pascal
Curated by ChEMBL
Universite Blaise Pascal
Curated by ChEMBL
Affinity DataKi: 6.04nMAssay Description:Displacement of [3H]SYM2081 from rat recombinant iGluR6More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 4(Homo sapiens (Human))
Allelix Biopharmaceuticals, Inc.
Curated by PDSP Ki Database
Allelix Biopharmaceuticals, Inc.
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 4(Rattus norvegicus)
Universite Blaise Pascal
Curated by ChEMBL
Universite Blaise Pascal
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity to rat cloned KA1 receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor 3(Homo sapiens (Human))
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
TargetGlutamate receptor 3(Homo sapiens (Human))
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database