null
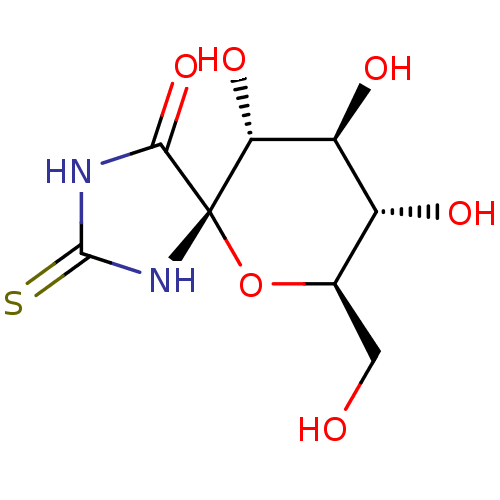
SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O
InChI Key InChIKey=OEWLGQKSTDZKFN-WWHASAIZSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50263769
Found 12 hits for monomerid = 50263769
TargetGlycogen phosphorylase, brain form(Homo sapiens (Human))
University of Debrecen
Curated by ChEMBL
University of Debrecen
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Inhibitory activity against muscle Glycogen Phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Competitive inhibition of rabbit skeletal muscle glycogen phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMpH: 6.8Assay Description:Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8More data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Oryctolagus cuniculus (rabbit))
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Inhibition of rabbit muscle glycogen phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
Lajos Kossuth University
Curated by ChEMBL
Lajos Kossuth University
Curated by ChEMBL
Affinity DataKi: 5.10E+3nMAssay Description:Inhibitor constant was measured against Muscle phosphorylase b in rabbitMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Rattus norvegicus)
Lajos Kossuth University
Curated by ChEMBL
Lajos Kossuth University
Curated by ChEMBL
Affinity DataKi: 7.00E+3nMAssay Description:Inhibitor constant was measured against Liver phosphorylase b in ratMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, brain form(Homo sapiens (Human))
University of Debrecen
Curated by ChEMBL
University of Debrecen
Curated by ChEMBL
Affinity DataKi: 7.00E+3nMAssay Description:Inhibitory activity against liver Glycogen Phosphorylase bMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, muscle form(Homo sapiens (Human))
Lajos Kossuth University
Curated by ChEMBL
Lajos Kossuth University
Curated by ChEMBL
Affinity DataKi: 1.09E+4nMAssay Description:Inhibitor constant was measured against Muscle phosphorylase a in rabbitMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
University of Debrecen
Curated by ChEMBL
University of Debrecen
Curated by ChEMBL
Affinity DataKi: 1.09E+4nMAssay Description:Inhibitory activity against muscle Glycogen Phosphorylase aMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
University of Debrecen
Curated by ChEMBL
University of Debrecen
Curated by ChEMBL
Affinity DataKi: 2.98E+4nMAssay Description:Inhibitory activity against liver Glycogen Phosphorylase aMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Rattus norvegicus)
Lajos Kossuth University
Curated by ChEMBL
Lajos Kossuth University
Curated by ChEMBL
Affinity DataKi: 2.98E+4nMAssay Description:Inhibitor constant was measured against Liver phosphorylase a in ratMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Rattus norvegicus)
Lajos Kossuth University
Curated by ChEMBL
Lajos Kossuth University
Curated by ChEMBL
Affinity DataKi: 2.98E+4nMAssay Description:Inhibition of rat liver glycogen phosphorylaseMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)