null
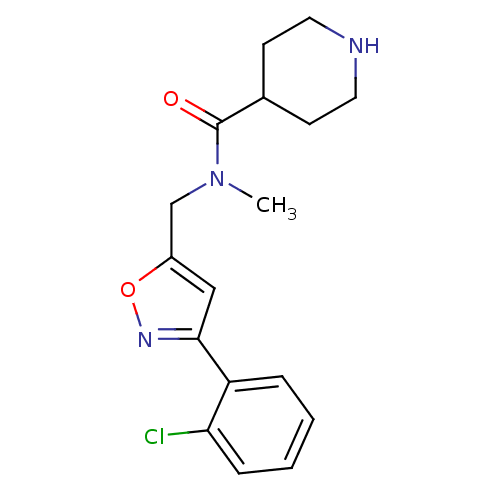
SMILES CN(Cc1cc(no1)-c1ccccc1Cl)C(=O)C1CCNCC1
InChI Key InChIKey=CYVLVDMCSIBANZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50264882
Found 3 hits for monomerid = 50264882
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 350nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human alpha3beta4 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human alpha3beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair