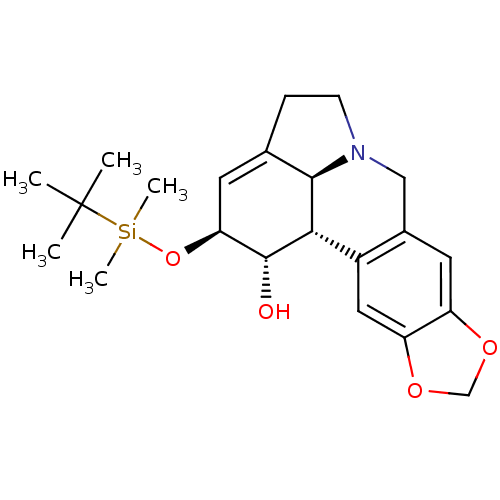
null
SMILES CC(C)(C)[Si](C)(C)O[C@H]1C=C2CCN3Cc4cc5OCOc5cc4[C@@H]([C@@H]23)[C@@H]1O
InChI Key InChIKey=RLYPONLPAHPYMG-UWHLTILDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50293600
Found 2 hits for monomerid = 50293600
Affinity DataKi: 260nMAssay Description:Inhibition of human CYP3A4 assessed as biotransformation of 7-benzyloxyquinolineMore data for this Ligand-Target Pair
Affinity DataKi: 860nMAssay Description:Inhibition of AChE by Ellman's assayMore data for this Ligand-Target Pair