null
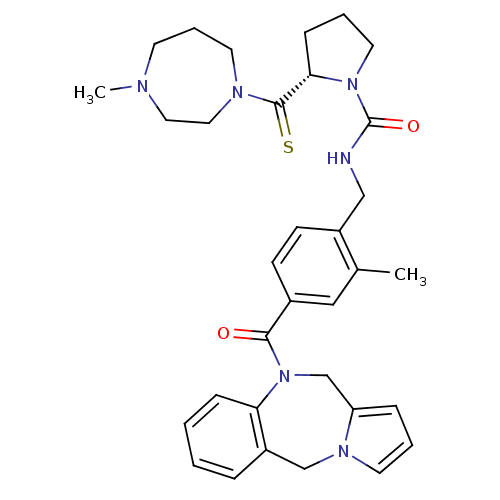
SMILES CN1CCCN(CC1)C(=S)[C@@H]1CCCN1C(=O)NCc1ccc(cc1C)C(=O)N1Cc2cccn2Cc2ccccc12
InChI Key InChIKey=XTIWITBMTVGYLG-PMERELPUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50307111
Found 3 hits for monomerid = 50307111
TargetVasopressin V1a receptor(Homo sapiens (Human))
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V1a receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:Displacement of [3H]AVP from human oxytocin receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair