null
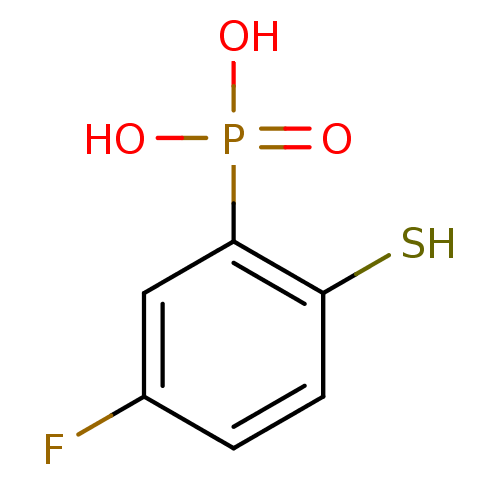
SMILES OP(O)(=O)c1cc(F)ccc1S
InChI Key InChIKey=RDAVYJKCJHOMSD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50322611
Found 3 hits for monomerid = 50322611
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 4.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair