null
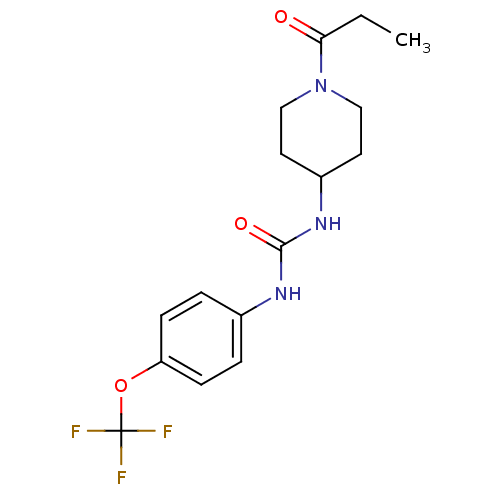
SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1
InChI Key InChIKey=AAJMQTLFRTZCJK-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50327809
Found 17 hits for monomerid = 50327809
Affinity DataKi: 0.910nMAssay Description:FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-...More data for this Ligand-Target Pair
Affinity DataKi: 0.910nMAssay Description:Binding affinity to purified recombinant human sEH by FRET-displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.910nMAssay Description:Any of a number of standard assays for determining epoxide hydrolase activity can be used to determine inhibition of sEH. For example, suitable assay...More data for this Ligand-Target Pair
Affinity DataKi: 0.910nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assayMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:The enzyme also can be detected based on the binding of specific ligands to the catalytic site which either immobilize the enzyme or label it with a ...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:The enzyme also can be detected based on the binding of specific ligands to the catalytic site which either immobilize the enzyme or label it with a ...More data for this Ligand-Target Pair
TargetEpoxide hydrolase 1(Felis catus (Cat) (Felis silvestris catus))
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA
US Patent
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA
US Patent
Affinity DataIC50: 400nMAssay Description:The enzyme also can be detected based on the binding of specific ligands to the catalytic site which either immobilize the enzyme or label it with a ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human C-terminal sEH (222 to 555 residues) expressed in Escherichia coli BL21-DE3 using PHOME as substrate assessed as redu...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase assessed as cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxyran-2-yl)methyl] carb...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Mus musculus (Mouse))
Department of Entomology and University of California Davis Cancer Center
Curated by ChEMBL
Department of Entomology and University of California Davis Cancer Center
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of mouse recombinant soluble epoxide hydrolase by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
University of California Davis
Curated by ChEMBL
University of California Davis
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of rat soluble epoxide hydrolase using [3H]-t-DPPO as a substrate by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMpH: 7.0Assay Description:IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Mus musculus (Mouse))
Department of Entomology and University of California Davis Cancer Center
Curated by ChEMBL
Department of Entomology and University of California Davis Cancer Center
Curated by ChEMBL
Affinity DataIC50: 2.80nMpH: 7.0Assay Description:IC50 values were determined using a sensitive fluorescent based assay (Anal. Biochem. 2005, 343, 66-75). Cyano(2-methoxynaphthalen-6-yl)methyl trans-...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)