null
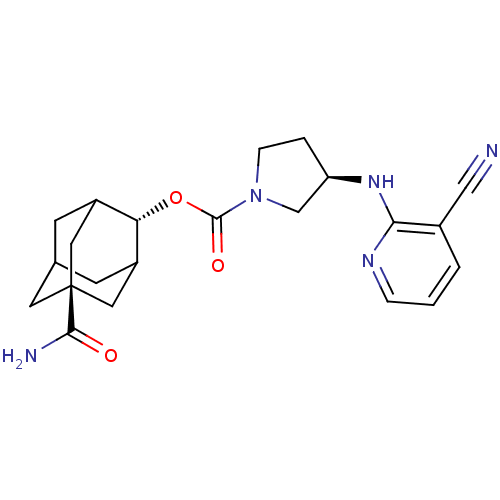
SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2
InChI Key InChIKey=BNIGHFUMARRMFX-GPJGMNBASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50329315
Found 9 hits for monomerid = 50329315
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.870nMAssay Description:Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Target3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 2(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of 3betaHSD2More data for this Ligand-Target Pair
Affinity DataIC50: 1.71E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase type 2(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7.35E+3nMAssay Description:Inhibition of 11betaHSD2More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader in presenc...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate readerMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 2(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of 17betaHSD2More data for this Ligand-Target Pair