null
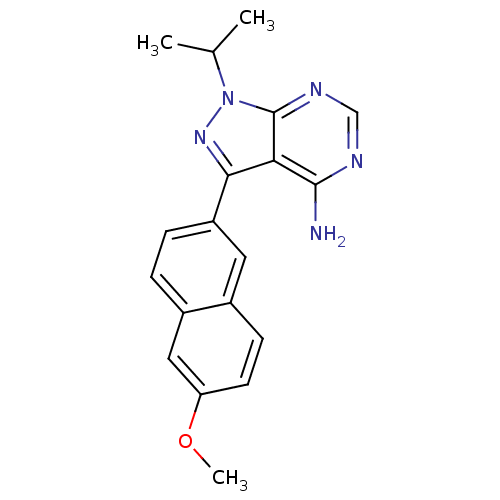
SMILES COc1ccc2cc(ccc2c1)-c1nn(C(C)C)c2ncnc(N)c12
InChI Key InChIKey=YTSDNPLOCDVGNB-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 27 hits for monomerid = 50345739
Found 27 hits for monomerid = 50345739
Affinity DataKi: 750nMAssay Description:Inhibition of human ABL by radiometric assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataKi: 770nMAssay Description:Inhibition of human SRC by radiometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of human LCK using Ac-EIYGEFKKK-OH as substrate after 60 minsMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human p38alpha using myelin basic protein as substrate after 180 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human EPHA3 using myelin basic protein as substrate after 120 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of human CSK using Ac-KKKKEEIYFFF-OH as substrate after 180 minsMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 510nMAssay Description:Inhibition of human EGFR using poly glu-Tyr as substrate after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Inhibition of human ABL using EAIYAAPFAKKK-OH as substrate by phosphorimaging methodMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Inhibition of human tyrosine kinases.More data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Inhibition of human tyrosine kinases.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase HCK(Homo sapiens (Human))
University of Washington Through its Center for Commercialization
US Patent
University of Washington Through its Center for Commercialization
US Patent
Affinity DataIC50: 375nMAssay Description:Inhibition of human tyrosine kinases.More data for this Ligand-Target Pair
TargetCalcium/calmodulin dependent protein kinase with a kinas domain and 4 calmodulin-like EF hands(Cryptosporidium parvum (strain Iowa II))TBA
Affinity DataIC50: 5nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetCalcium-dependent protein kinase 1(Cryptosporidium parvum)
University of Washington Through its Center for Commercialization
US Patent
University of Washington Through its Center for Commercialization
US Patent
Affinity DataIC50: 5nMAssay Description:Two types of enzyme assays were developed to follow TgCDPK1 activity, a radiometric scintillation proximity assay measured the labeled γ-phospha...More data for this Ligand-Target Pair
TargetCalmodulin-domain protein kinase 1(Toxoplasma gondii)
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetCalmodulin-domain protein kinase 1, putative(Cryptosporidium parvum (strain Iowa II))
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
Affinity DataIC50: 5nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 576nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase HCK(Homo sapiens (Human))
University of Washington Through its Center for Commercialization
US Patent
University of Washington Through its Center for Commercialization
US Patent
Affinity DataIC50: 375nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetCalcium/calmodulin dependent protein kinase with a kinas domain and 4 calmodulin-like EF hands(Cryptosporidium parvum (strain Iowa II))TBA
Affinity DataIC50: 5nMAssay Description:Kinase phosphorylation reactions were performed in a buffered medium containing 20 mM HEPES pH 7.5 (KOH), 0.1% BSA, 10 mM MgCl2, 1 mM EGTA (pH 7.2), ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Kinase phosphorylation reactions were performed in a buffered medium containing 20 mM HEPES pH 7.5 (KOH), 0.1% BSA, 10 mM MgCl2, 1 mM EGTA (pH 7.2), ...More data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Kinase phosphorylation reactions were performed in a buffered medium containing 20 mM HEPES pH 7.5 (KOH), 0.1% BSA, 10 mM MgCl2, 1 mM EGTA (pH 7.2), ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 770nMAssay Description:Kinase phosphorylation reactions were performed in a buffered medium containing 20 mM HEPES pH 7.5 (KOH), 0.1% BSA, 10 mM MgCl2, 1 mM EGTA (pH 7.2), ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 670nMAssay Description:Inhibition of human SRC using Ac-EIYGEFKKK-OH as substrate after 60 mins by phosphorimaging methodMore data for this Ligand-Target Pair
TargetCalmodulin-domain protein kinase 1(Toxoplasma gondii)
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition of Toxoplasma gondii recombinant N-terminal hexahistidine tagged CDPK1 expressed in Escherichia coli using syntide-2 as substrate after 90...More data for this Ligand-Target Pair
TargetCalmodulin-domain protein kinase 1(Toxoplasma gondii)
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO
US Patent
Affinity DataIC50: 6nMAssay Description:Most known kinase inhibitors bind in the ATP-binding pocket of the active site19,20. These inhibitors exploit many of the same hydrophobic contacts a...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)