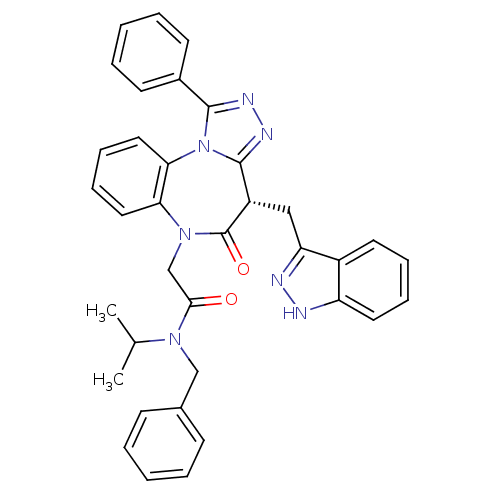
null
SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2n[nH]c3ccccc23)C1=O
InChI Key InChIKey=NQJDEFGZTFBIOH-NDEPHWFRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50380724
Found 2 hits for monomerid = 50380724
Affinity DataIC50: 38.1nMAssay Description:Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 380nMAssay Description:Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati...More data for this Ligand-Target Pair