null
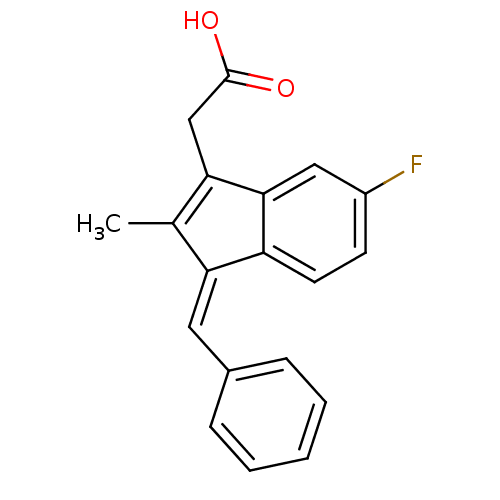
SMILES CC1=C(CC(O)=O)c2cc(F)ccc2\C1=C/c1ccccc1
InChI Key InChIKey=MVHHIVKPIOEVNA-SXGWCWSVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50430575
Found 3 hits for monomerid = 50430575
Affinity DataIC50: 1.07E+4nMAssay Description:Displacement of [3H]-9-cis-RA from human GST-tagged N-terminal truncated RXRalpha after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKd: 7.94E+3nMAssay Description:Binding affinity to GAL4-DBD-fused PPARgamma ligand binding domain (unknown origin) expressed in HEK293T cells by spectra-fluorophotometry analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 2.78E+3nMAssay Description:Partial agonist activity at GAL4 DBD-fused PPARgamma LBD (unknown origin) expressed in pG5 luc and pBIND transfected HEK293T cells assessed as transc...More data for this Ligand-Target Pair