null
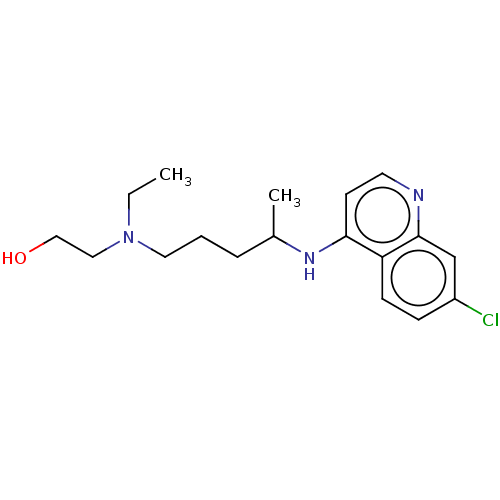
SMILES CCN(CCO)CCCC(C)Nc1ccnc2cc(Cl)ccc12
InChI Key InChIKey=XXSMGPRMXLTPCZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 22 hits for monomerid = 50467780
Found 22 hits for monomerid = 50467780
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 126nMAssay Description:Displacement of [3H]-pentazocin from the Sigma1 receptorMore data for this Ligand-Target Pair
TargetSigma intracellular receptor 2(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H]-DTG from the Sigma2 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cellsMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 4.79E+3nMAssay Description:Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNBMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 5.13E+3nMAssay Description:Activity of compound against Muscarinic acetylcholine receptor M2 (CHRM2) by displacement of 3H-QNBMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 5.50E+3nMAssay Description:Activity of compound against Muscarinic acetylcholine receptor M4 (CHRM4) by displacement of 3H-QNBMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 6.46E+3nMAssay Description:Activity of compound against Alpha-2A (ADRA2A) adrenergic receptor by displacement of [3H]-rauwolscineMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 1.55E+4nMAssay Description:Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNBMore data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 1.58E+4nMAssay Description:Activity of compound against Alpha 2C (ADRA2C) adrenergic receptor by displacement of [3H]-rauwolscineMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 2.00E+4nMAssay Description:Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNBMore data for this Ligand-Target Pair
TargetAlpha-2B adrenergic receptor(Homo sapiens (Human))
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
Affinity DataKi: 7.24E+4nMAssay Description:Activity of compound against Alpha 2B (ADRA2B) adrenergic receptor by displacement of [3H]-rauwolscineMore data for this Ligand-Target Pair
Affinity DataKi: 9.23E+4nMAssay Description:Inhibition of Zika virus NS2B-NS3 proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Antagonist activity at TLR7 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.58E+6nMAssay Description:This is a review article. Please point to the original journal.More data for this Ligand-Target Pair
Affinity DataIC50: 8.60E+3nMAssay Description:Antagonist activity at human TLR7 expressed in HEK-Blue cells assessed as inhibition of CL264-induced NFkappaB activation by measuring reduction in S...More data for this Ligand-Target Pair
TargetReplicase polyprotein 1ab(Human SARS coronavirus (SARS-CoV) (Severe acute re...)
University of Bonn
University of Bonn
Affinity DataEC50: 7.96E+3nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataEC50: 8.27E+3nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataEC50: 740nMAssay Description:This is a review article.More data for this Ligand-Target Pair
In DepthDetails
TargetToll-like receptor 9(Homo sapiens (Human))
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
CSIR-Indian Institute of Chemical Biology
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Antagonist activity at human TLR9 expressed in HEK-Blue cells assessed as reduction in CpGB-induced NF-kappaB levels after 24 hrs by spectrophotometr...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Antagonist activity at human TLR7 expressed in HEK-Blue cells assessed as reduction in CL264-induced NF-kappaB levels after 24 hrs by spectrophotomet...More data for this Ligand-Target Pair