null
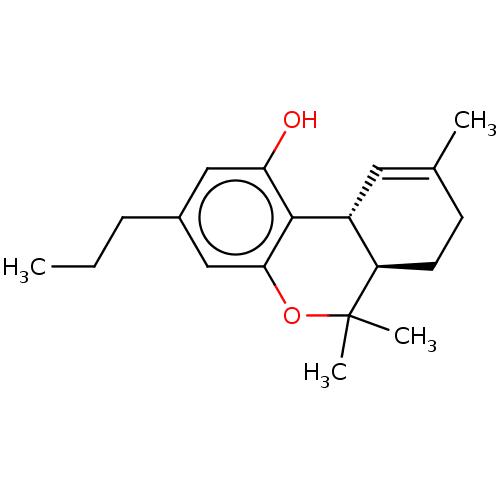
SMILES [H][C@@]12C=C(C)CC[C@@]1([H])C(C)(C)Oc1cc(CCC)cc(O)c21
InChI Key InChIKey=ZROLHBHDLIHEMS-HUUCEWRRSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 35 hits for monomerid = 50511105
Found 35 hits for monomerid = 50511105
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]-CP-55,940 from recombinant human CB1R expressed in HEK293 cell membranes after 90 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]-CP-55,940 from recombinant human CB2R expressed in HEK293 cell membranes after 90 minsMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Displacement of [3H]-CP55940 from recombinant human CB2 receptor expressed in CHO cell membranes measured after 60 mins by liquid scintillation count...More data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Displacement of [3H]-CP55940 from CB1 receptor in mouse whole brain membrane measured after 60 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Binding affinity to human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Displacement of [3H]-CP-55,940 from recombinant human CB2R expressed in HEK293 cell membranes after 90 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]-CP-55,940 from recombinant human CB1R expressed in HEK293 cell membranes after 90 minsMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Concordia University Wisconsin
Curated by ChEMBL
Concordia University Wisconsin
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant human NAAA expressed in HEK293 cells using [14C]-N-palmitoylethanolamine as substrate measured after 30 mins by beta-counti...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Concordia University Wisconsin
Curated by ChEMBL
Concordia University Wisconsin
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of FAAH in rat brain membranes using [14C]-AEA as substrate measured after 30 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetDiacylglycerol lipase-alpha(Homo sapiens (Human))
Concordia University Wisconsin
Curated by ChEMBL
Concordia University Wisconsin
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of recombinant human DAGLalpha expressed in COS7 cells using [14C]-oleoyl-2-arachidonoyl-glycerol as substrate measured after 20 mins by b...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)