null
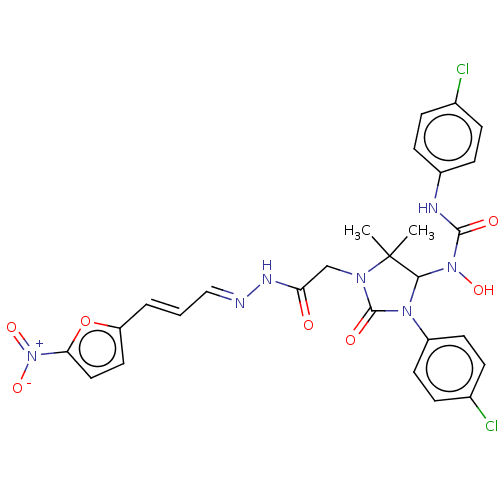
SMILES CC1(C)C(N(O)C(=O)Nc2ccc(Cl)cc2)N(C(=O)N1CC(=O)N\N=C\C=C\c1ccc(o1)[N+]([O-])=O)c1ccc(Cl)cc1
InChI Key InChIKey=JTUXTPWYZXWOIB-LWWHHIEBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50514012
Found 3 hits for monomerid = 50514012
Affinity DataKd: 5.00E+3nMAssay Description:Binding affinity to p97 (unknown origin) by SPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi...More data for this Ligand-Target Pair