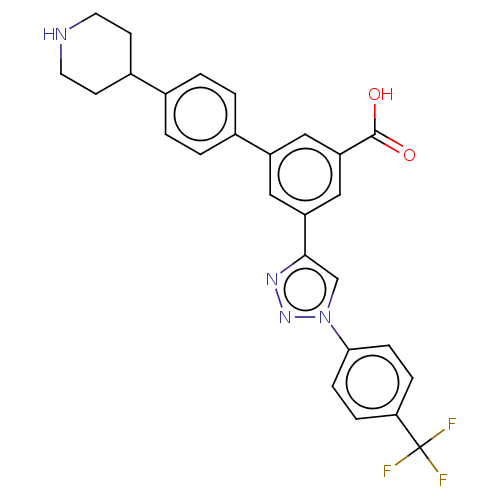
null
SMILES OC(=O)c1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)-c1cn(nn1)-c1ccc(cc1)C(F)(F)F
InChI Key InChIKey=WYKMWZDXNRNMFC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50541996
Found 13 hits for monomerid = 50541996
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 960nMAssay Description:Binding affinity to adrenergic alpha2A receptor (unknown origin)More data for this Ligand-Target Pair
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.48E+3nMAssay Description:Binding affinity to sigma 1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.71E+3nMAssay Description:Binding affinity to adrenergic alpha2C receptor (unknown origin)More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 3.79E+3nMAssay Description:Binding affinity to DOR (unknown origin)More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 3.79E+3nMAssay Description:Binding affinity to DOR (unknown origin)More data for this Ligand-Target Pair
TargetAlpha-2B adrenergic receptor(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 5.33E+3nMAssay Description:Binding affinity to adrenergic alpha2B receptor (unknown origin)More data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Antagonist activity at CB2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 246nMAssay Description:Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-...More data for this Ligand-Target Pair
TargetP2Y purinoceptor 14(Homo sapiens (Human))
Saint Louis University School of Medicine
Curated by ChEMBL
Saint Louis University School of Medicine
Curated by ChEMBL
Affinity DataIC50: 664nMAssay Description:Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at CB1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Antagonist activity at CB1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at CB2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 246nMAssay Description:Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3...More data for this Ligand-Target Pair