null
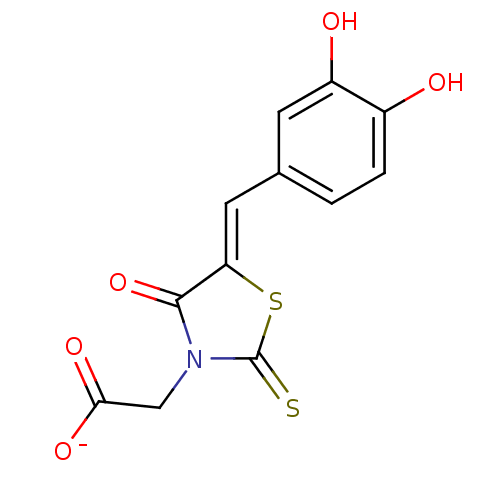
SMILES Oc1ccc(\C=C2/SC(=S)N(CC([O-])=O)C2=O)cc1O
InChI Key InChIKey=MNPZQUQRMCXSTL-WTKPLQERSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 59098
Found 3 hits for monomerid = 59098
Affinity DataKi: 26nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair