null
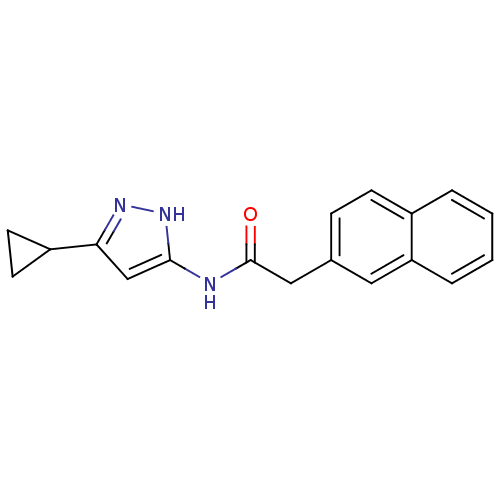
SMILES O=C(Cc1ccc2ccccc2c1)Nc1cc(n[nH]1)C1CC1
InChI Key InChIKey=RIGZCVNCFXYBEG-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 7108
Found 9 hits for monomerid = 7108
Affinity DataIC50: 37nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaantibodypediaGoogleScholar
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2WQ020RPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2WQ020RPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 92nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 114nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CDK4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in...More data for this Ligand-Target Pair