null
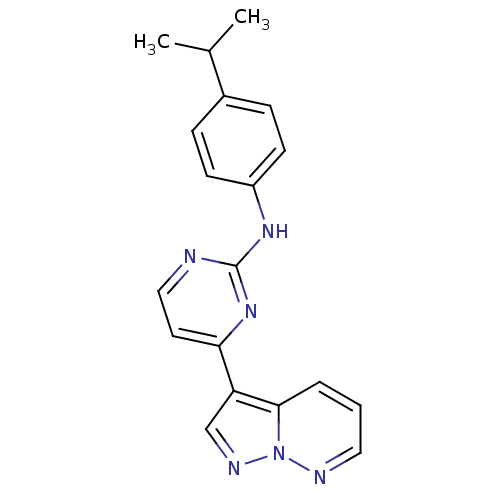
SMILES CC(C)c1ccc(Nc2nccc(n2)-c2cnn3ncccc23)cc1
InChI Key InChIKey=UODXTGBFBIFREQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 8131
Found 9 hits for monomerid = 8131
Affinity DataIC50: 16nMpH: 7.2 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 1.99E+3nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of CDK4 by radioactive glutathione plate-binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The compound was evaluated for its relative binding affinity against mutant N131A scytalone dehydratase; Relative KiMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 40nMAssay Description:Inhibition of human CDK2 by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Ability to displace [3H]glycine from strychnine-insensitive glycine receptorMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.10nMAssay Description:The compound was evaluated for its relative binding affinity against mutant Y50F scytalone dehydratase; Relative KiMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 16nMAssay Description:Inhibition of GSK3beta by scintillation proximity assayMore data for this Ligand-Target Pair