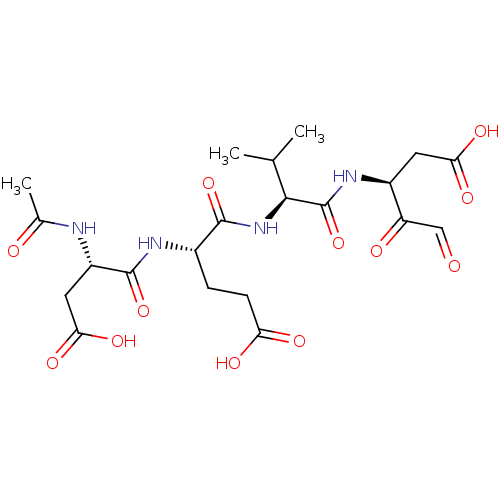
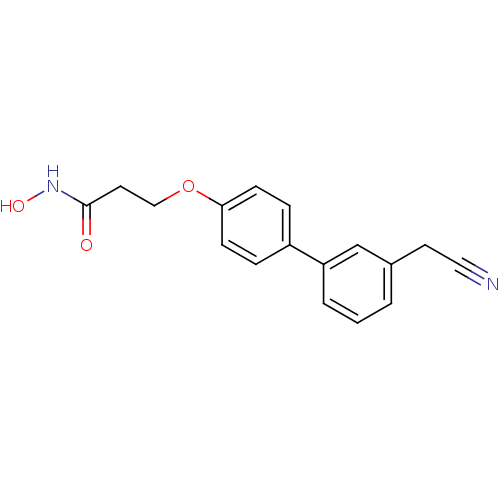
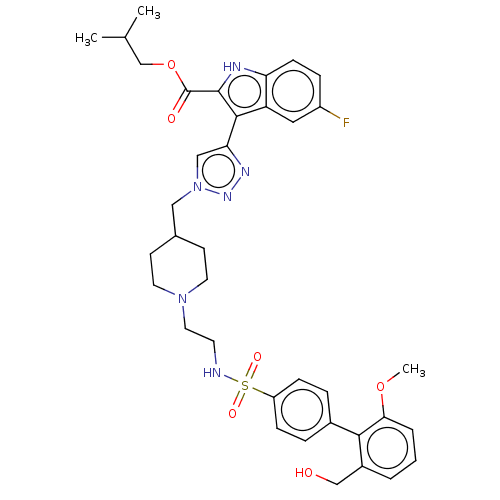
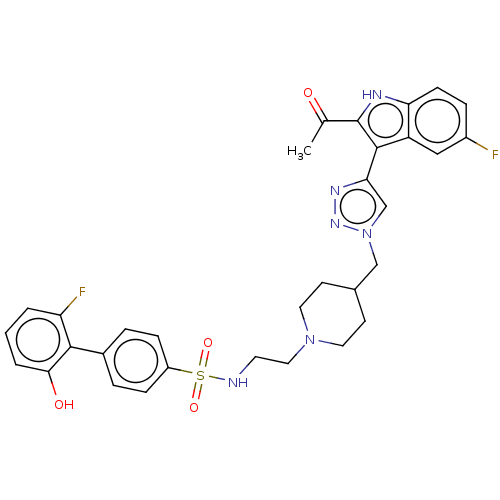
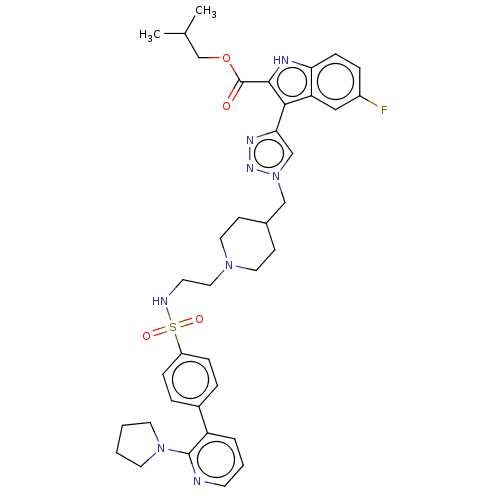
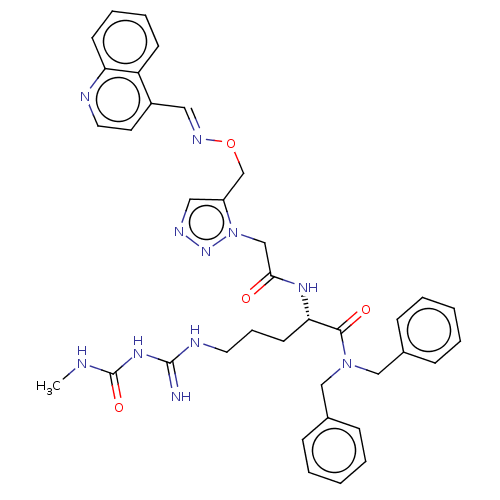
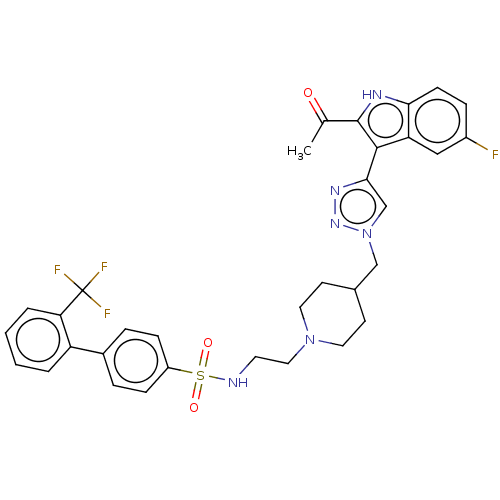
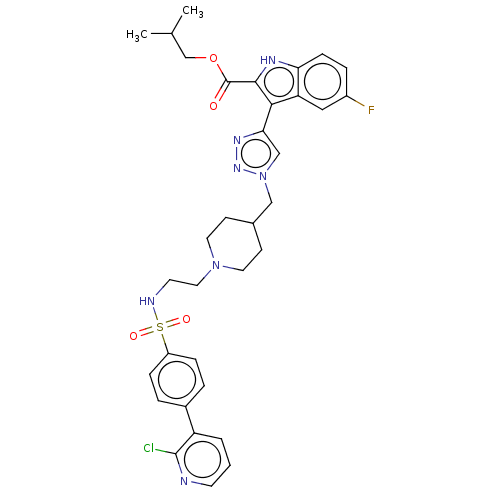
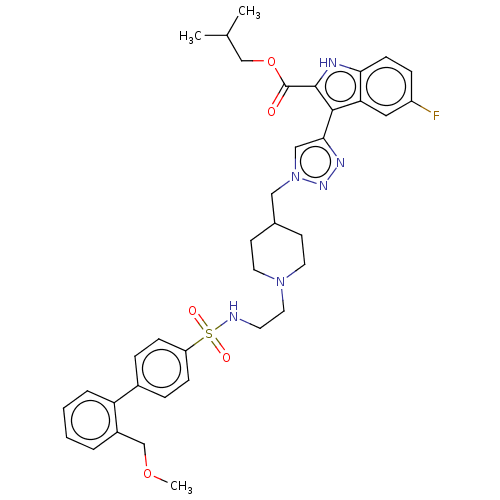
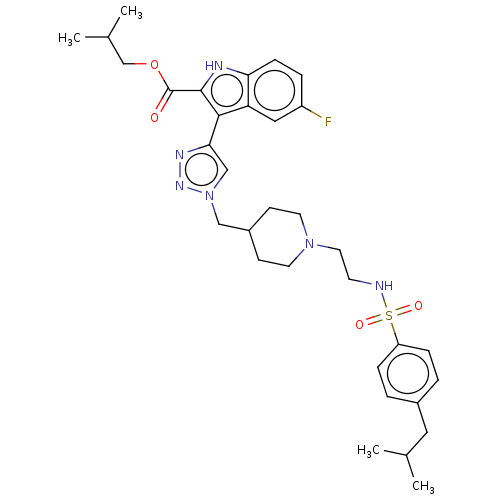
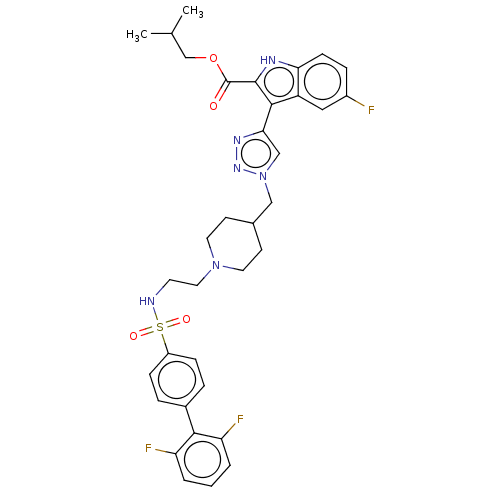
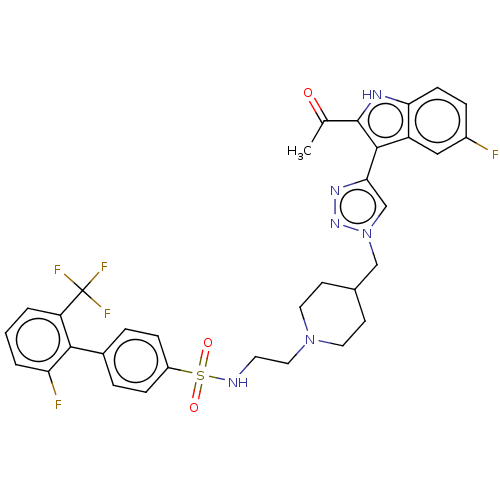
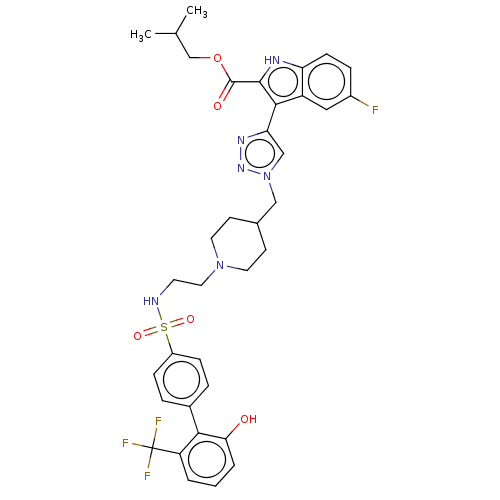
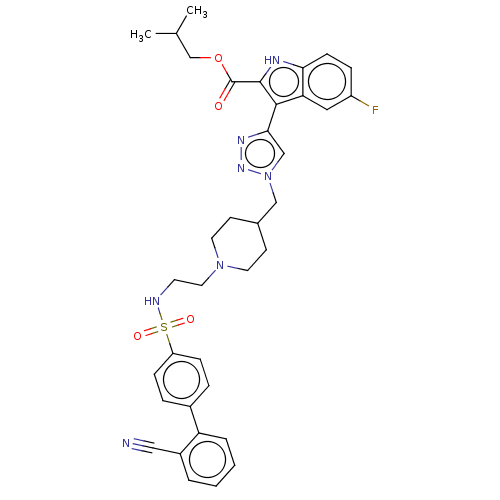
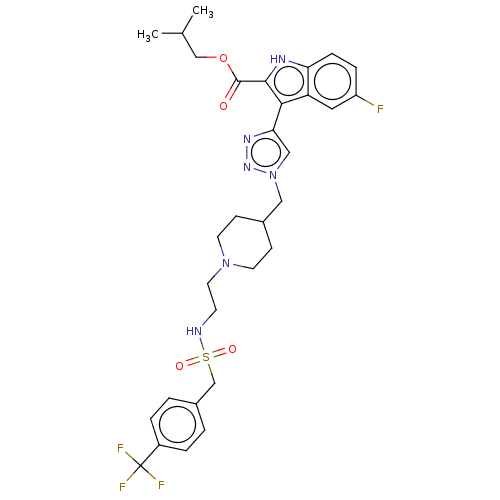
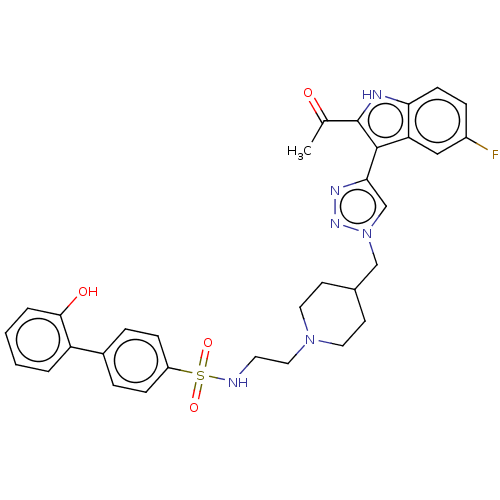
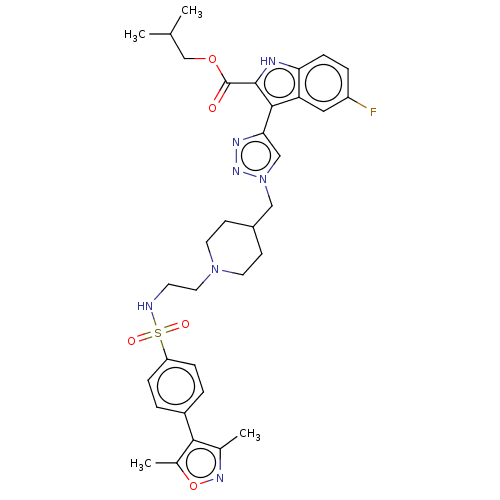
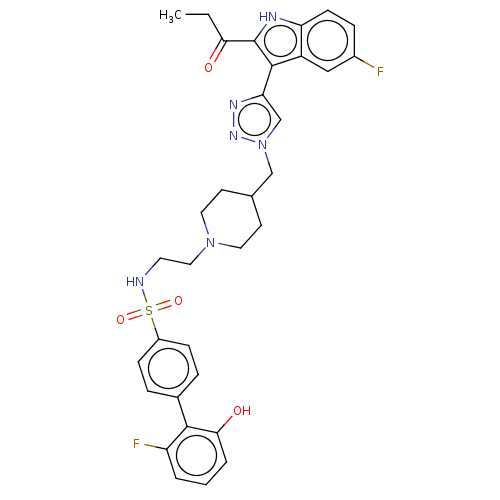
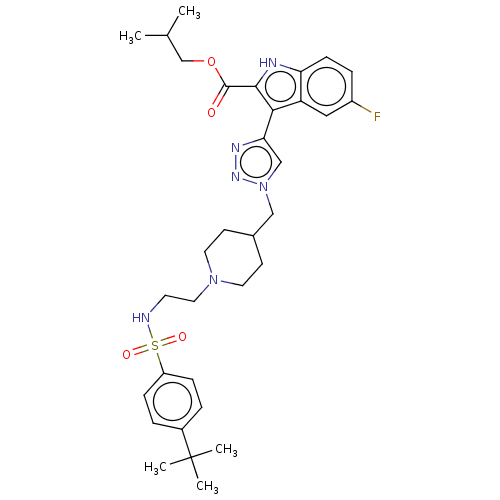
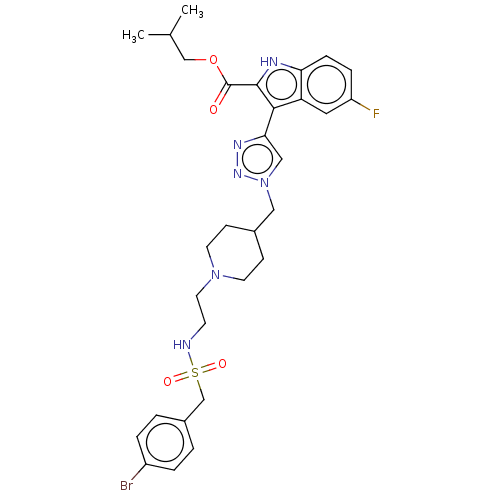
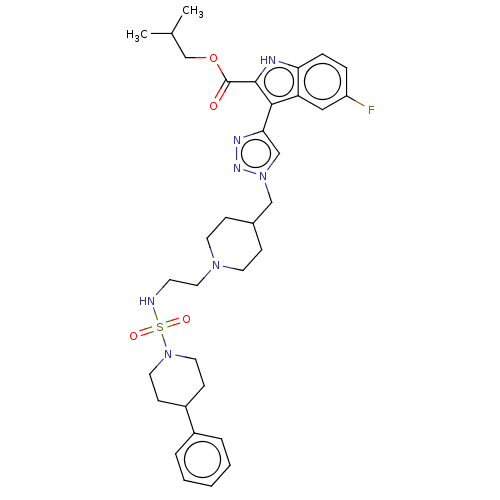
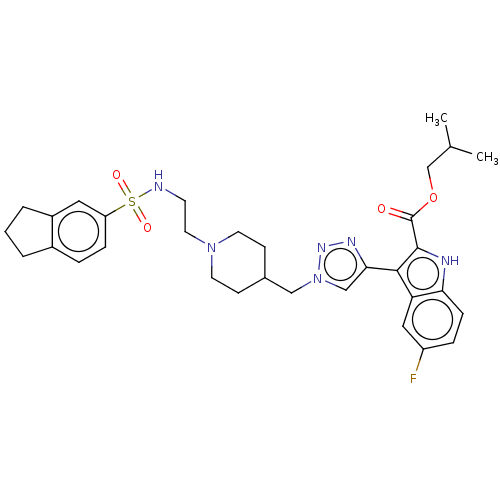
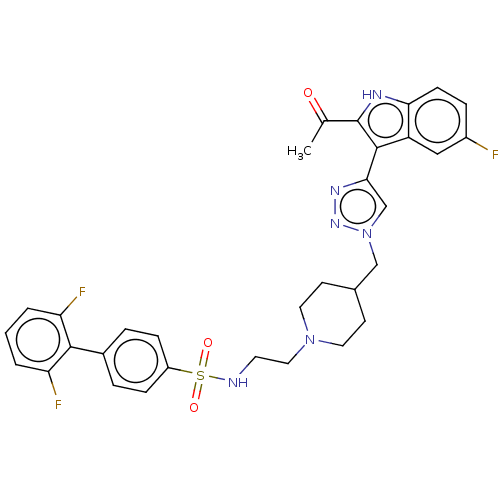
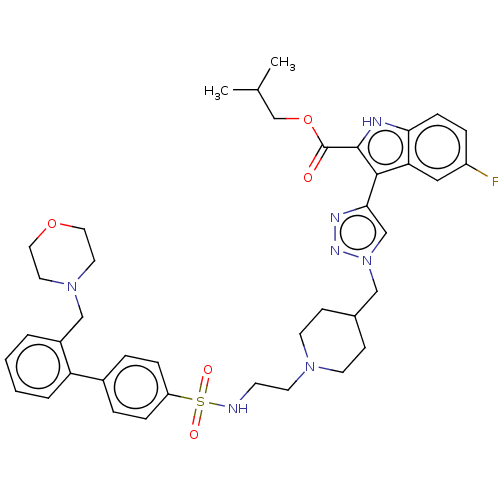
Affinity DataKi: <0.0100nMAssay Description:Inhibition of BCL2 (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: <0.5nMAssay Description:Inhibition of BCL2 (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
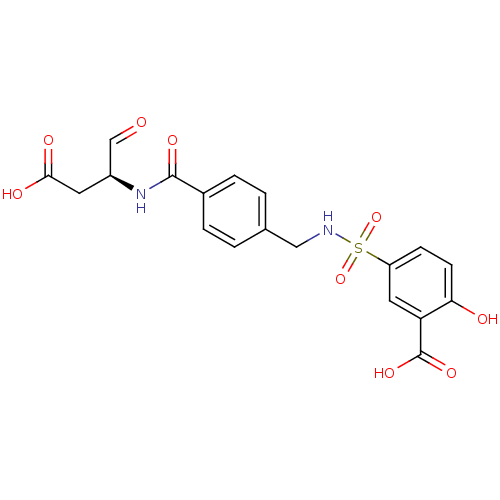
Affinity DataKi: 22nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate measured every 30 secs for 15 mins by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysisMore data for this Ligand-Target Pair
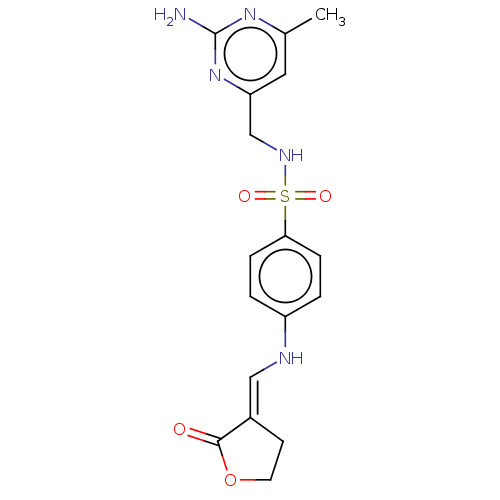
Affinity DataKi: 29nMAssay Description:Inhibition of human factor 10a using Boc-Leu-Gly-Arg-AMC as fluorogenic substrate measured after 10 mins by spectrofluorimetryMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibition of recombinant human caspase 3 using Ac-Asp-Glu-Val-Asp-AFC as substrate preincubated for 30 mins followed by substrate addition and measu...More data for this Ligand-Target Pair
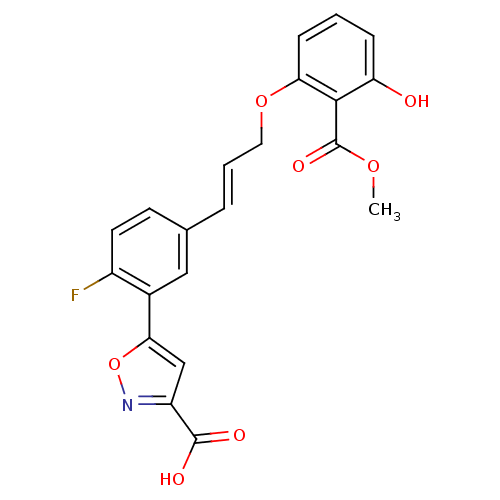
Affinity DataKi: 1.90E+3nMAssay Description:Displacement of biotin-labelled geldanamycin from human HSP90 (D9 to E236 residues) incubated for 3 hrs by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of human HSP90 (D9 to E236 residues) by fluorescence spectroscopyMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate measured every 30 secs for 15 mins by Michaelis-Menten plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+5nMAssay Description:Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Inhibition of human factor 10a using Boc-Leu-Gly-Arg-AMC as fluorogenic substrate measured after 10 mins by spectrofluorimetryMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+6nMAssay Description:Inhibition of human factor 10a using Boc-Leu-Gly-Arg-AMC as fluorogenic substrate measured after 10 mins by spectrofluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazineMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human NMT1 by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of MMP3 (unknown origin) using Ac-Pro-Leu-Gly-[2-mercapto-4-methyl-pentanoyl]-Leu-Gly-OEt as substrate incubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of Serratia marcescens chitinase BMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CDK2/Cyclin A (unknown origin) using histone H1 as substrate by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibition of Endothia parasitica endothiapepsin using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 64nMAssay Description:Inhibition of c-Src (unknown origin) using biotin-KVEKIGEGTYGVVYK as substrate by ELISAMore data for this Ligand-Target Pair


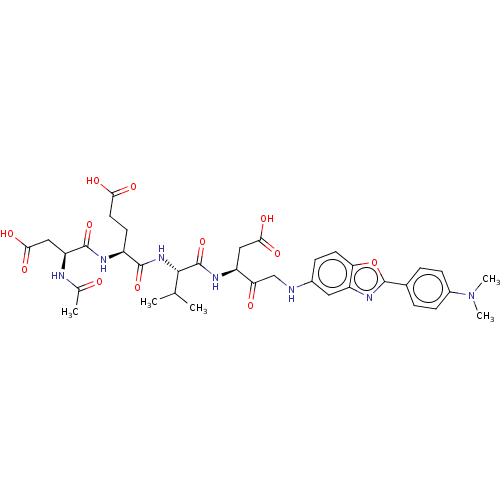

 3D Structure (crystal)
3D Structure (crystal)