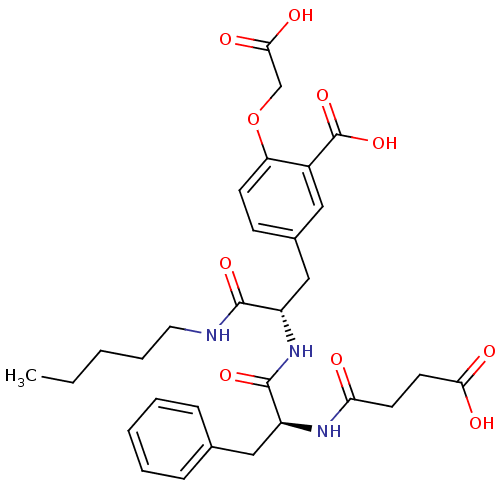
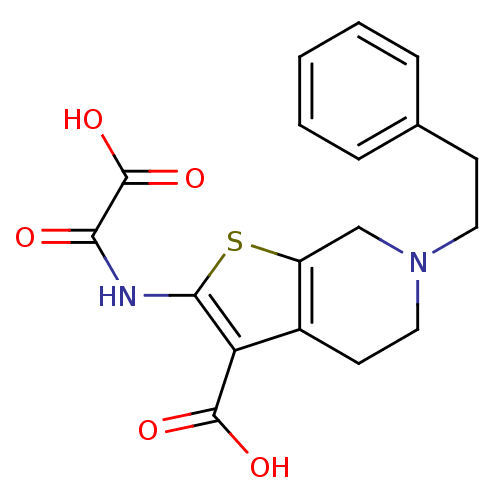
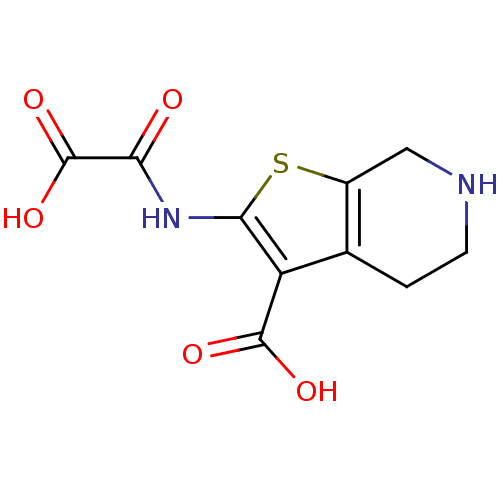
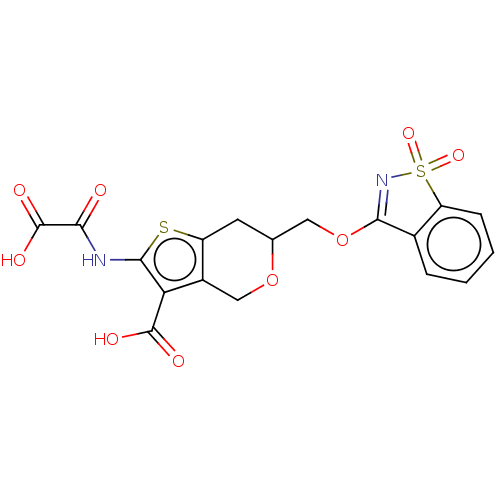
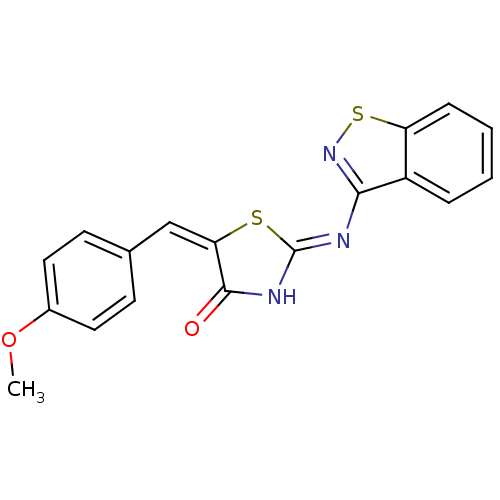
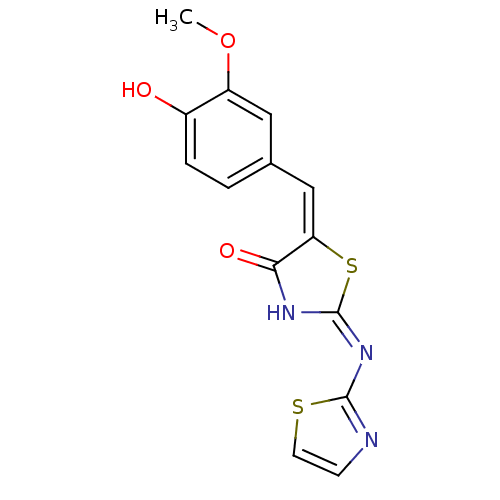
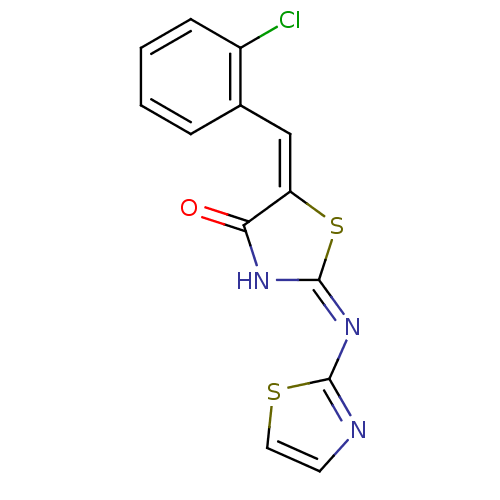
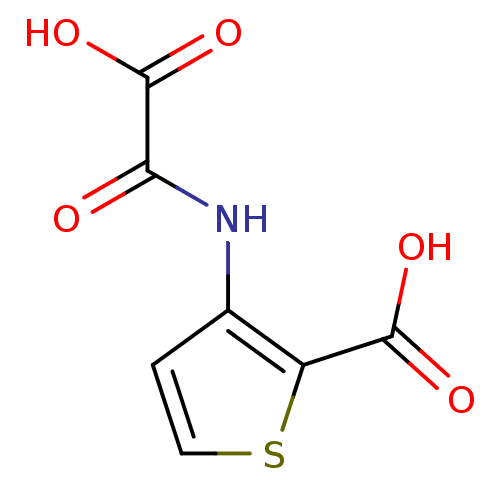
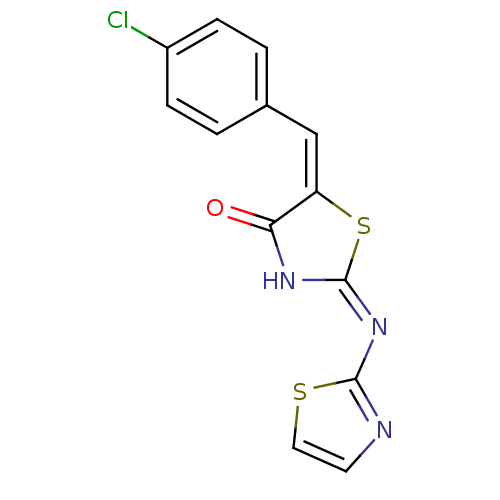
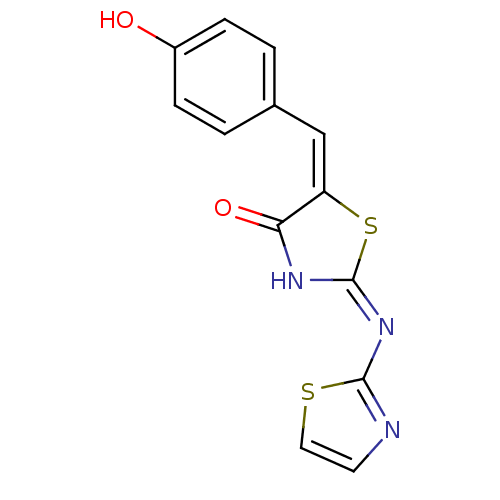
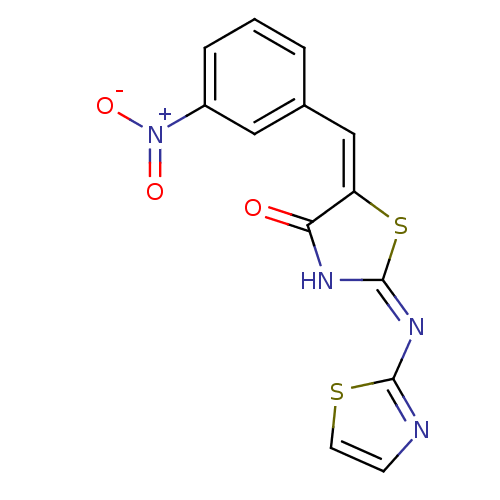
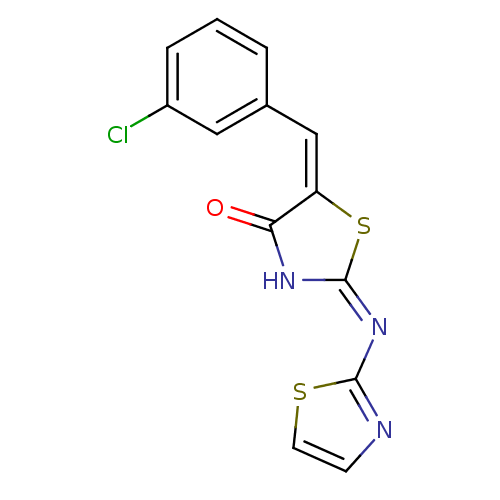
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
In DepthDetails
Affinity DataKi: 1.17E+4nM ΔG°: -28.1kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 1.17E+4nM ΔG°: -28.1kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 1.23E+4nM ΔG°: -28.0kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 1.29E+4nM ΔG°: -27.9kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 1.62E+4nM ΔG°: -27.3kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 1.81E+4nM ΔG°: -27.1kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 2.28E+4nM ΔG°: -26.5kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 2.56E+4nM ΔG°: -26.2kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 2.87E+4nM ΔG°: -25.9kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 3.23E+4nM ΔG°: -25.6kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Affinity DataKi: 5.45E+4nM ΔG°: -24.3kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 9.08E+4nM ΔG°: -23.1kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 1.03E+5nM ΔG°: -22.8kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataKi: 3.88E+5nM ΔG°: -19.5kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataKi: 4.74E+5nM ΔG°: -19.0kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 5.48E+5nM ΔG°: -18.6kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
Affinity DataKi: 1.09E+6nM ΔG°: -16.9kJ/molepH: 7.0 T: 2°CAssay Description:SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
Affinity DataIC50: 2.60nMAssay Description:Inhibition of recombinant human DYKDDDD-tagged EGFR (669 to 1210 residues) expressed in baculovirus expression system using poly-Glu-Tyr (4:1) as sub...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2NS0ZNJPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2NS0ZNJPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails