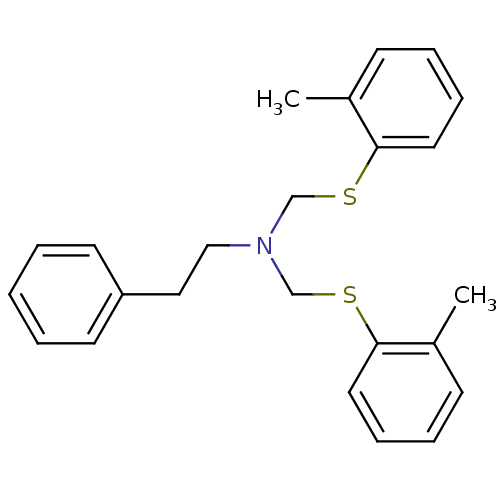
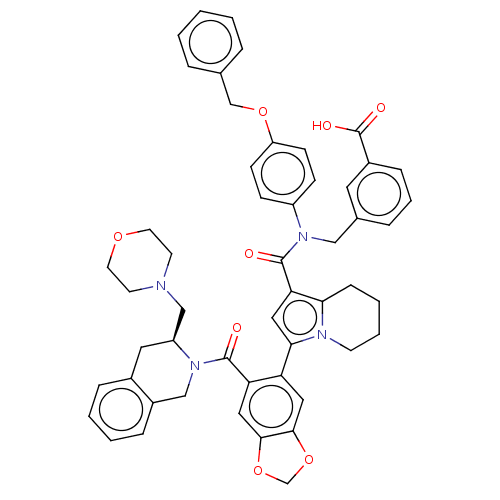
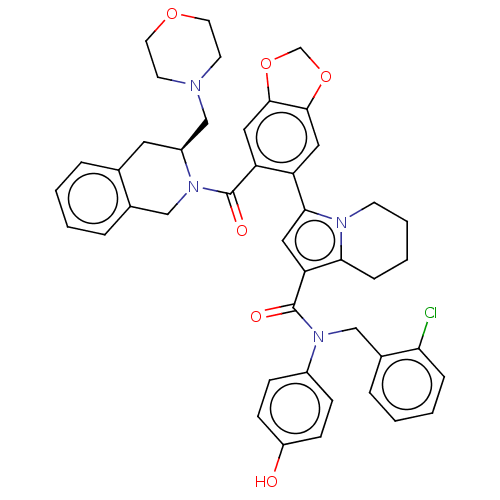
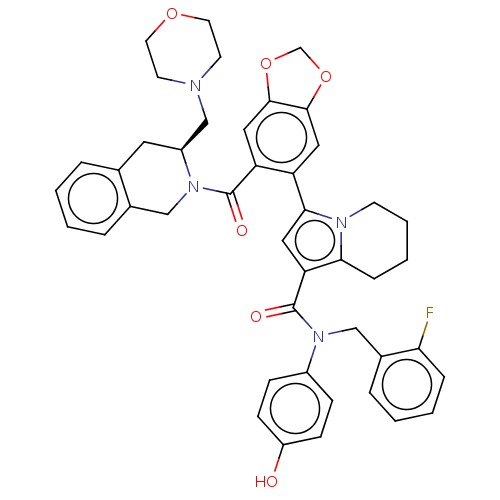
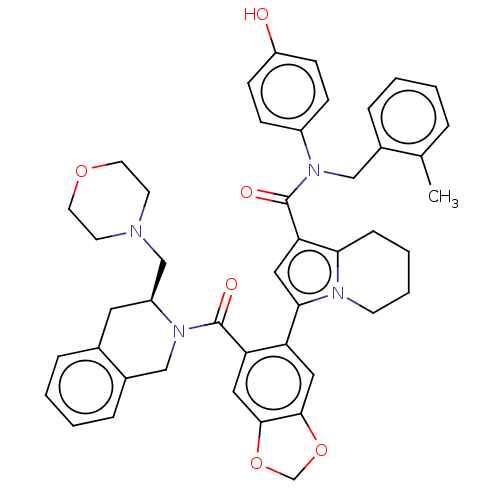
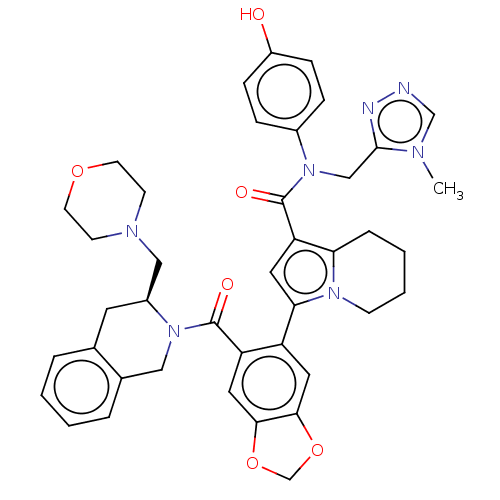
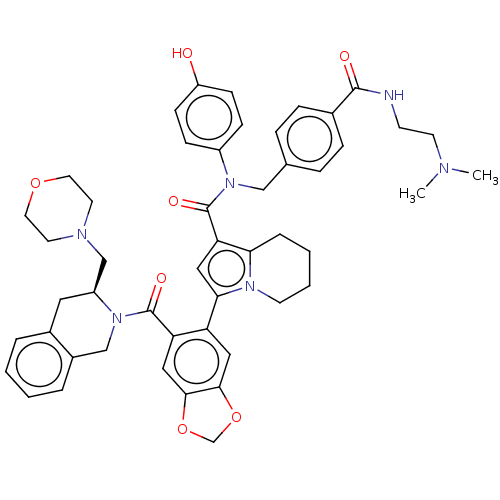
Affinity DataKi: 25nMAssay Description:Displacement of [125I-BDZ-2] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
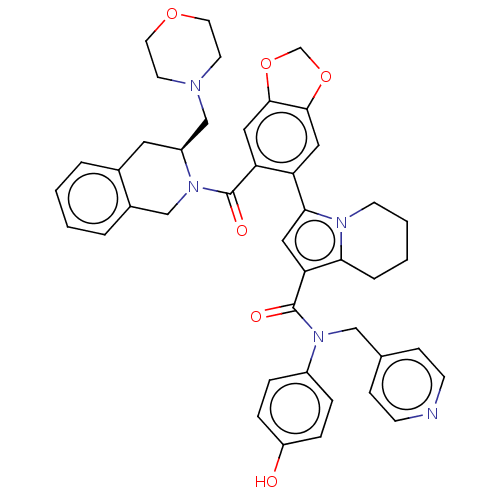
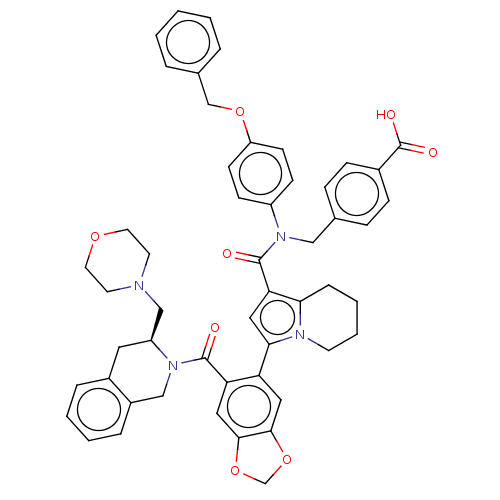
Affinity DataKi: 25nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
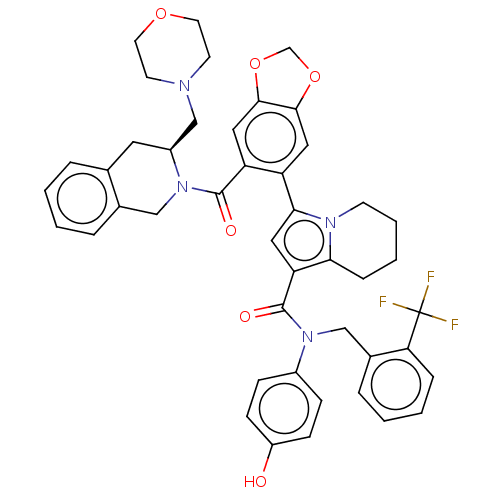
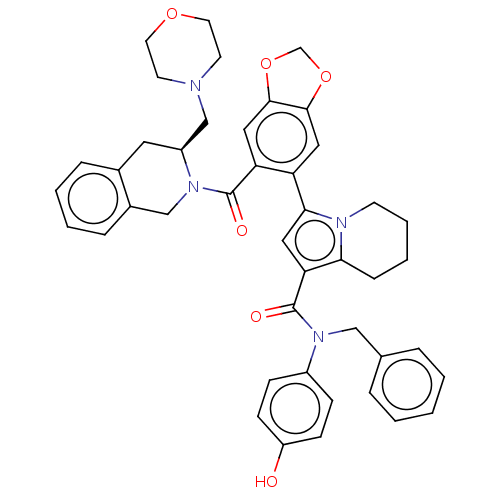
Affinity DataKi: 29nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t...More data for this Ligand-Target Pair
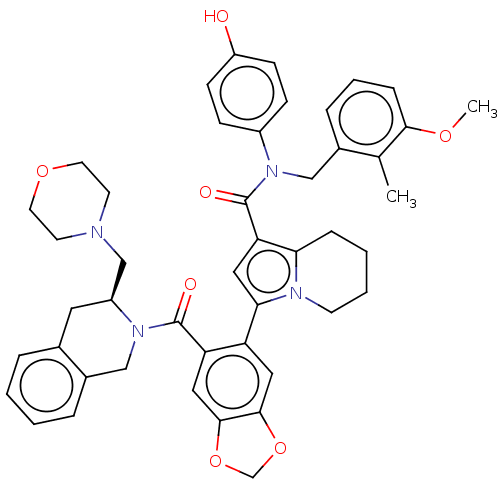
Affinity DataKi: 69nMAssay Description:Displacement of [125I-BDZ-2] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 71nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 166nMAssay Description:Displacement of [125I-BDZ-1] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Displacement of [125I-BDZ-2] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 214nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 214nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 275nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 427nMAssay Description:Displacement of [125I-BDZ-1] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 800nM IC50: 1.70E+3nMAssay Description:Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t...More data for this Ligand-Target Pair
Affinity DataKi: 912nMAssay Description:Displacement of [125I-BDZ-1] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: <1.00E+3nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: <1.00E+3nMAssay Description:Displacement of [125I-BDZ-1] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nMAssay Description:Displacement of [125I-BDZ-2] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 1.41E+3nMAssay Description:Displacement of [125I-CCK] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
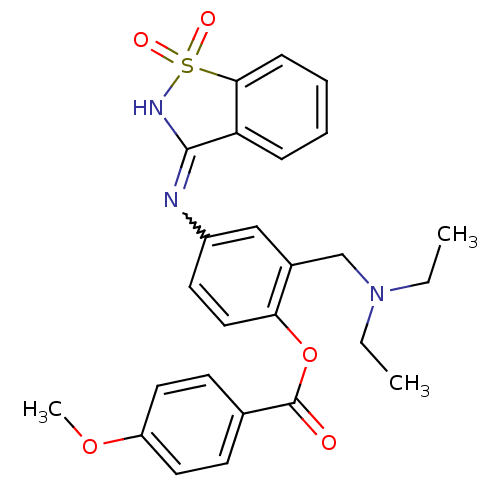
Affinity DataKi: 1.70E+3nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+3nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+3nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 7.80E+3nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 8.10E+3nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.13E+4nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.19E+4nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.09E+4nMAssay Description:Displacement of [125I]MCH from human MCHR1 expressed in CHO cell membranesMore data for this Ligand-Target Pair
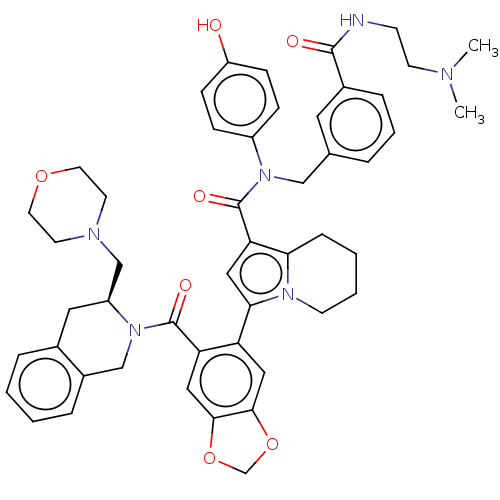
Affinity DataIC50: 0.813nMAssay Description:Displacement of [125I-CCK] from human CCK1R N2.61T mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Displacement of [125I-CCK] from human CCK1R W6.48A mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [125I-CCK] from human CCK2R H7.39L mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [125I-CCK] from human CCK1R N2.61T mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [125I-BDZ-2] from human CCK2R H7.39L mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Agonist activity at human CCK1R V3.36A mutant expressed in CHO cells assessed as intracellular calcium response by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Displacement of [125I-BDZ-1] from human CCK1R T3.28V, T3.29S mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20nMAssay Description:Displacement of [125I-BDZ-1] from human CCK1R T3.28V, T3.29S mutant expressed in CHO cells after 60 mins by scintillation counterMore data for this Ligand-Target Pair


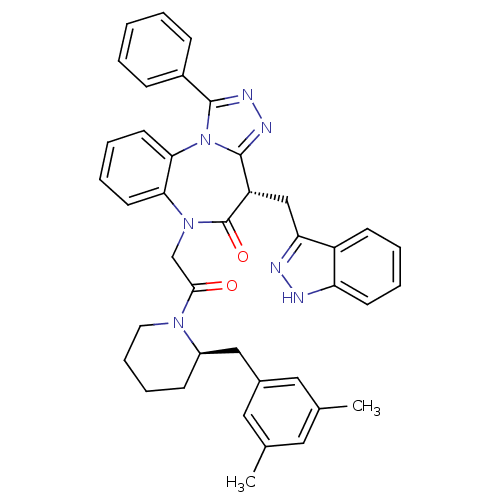


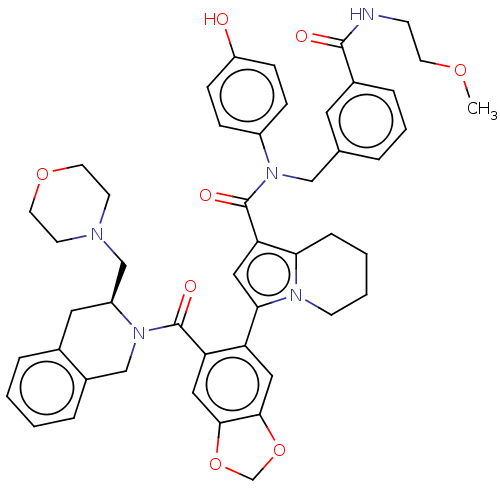
 3D Structure (crystal)
3D Structure (crystal)