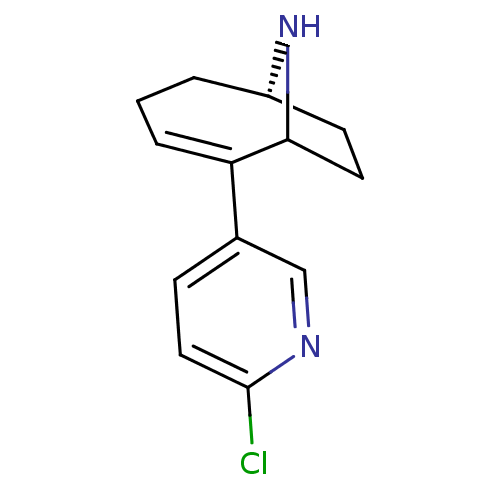
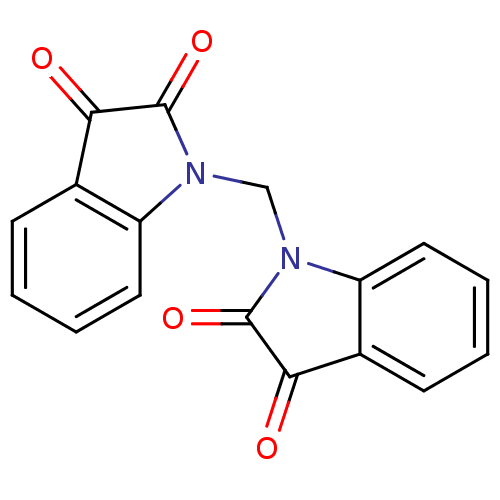
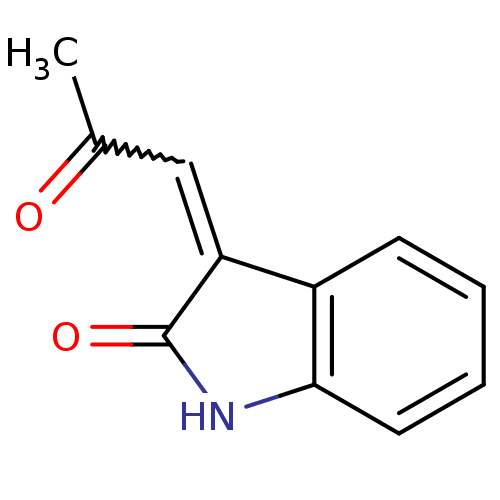
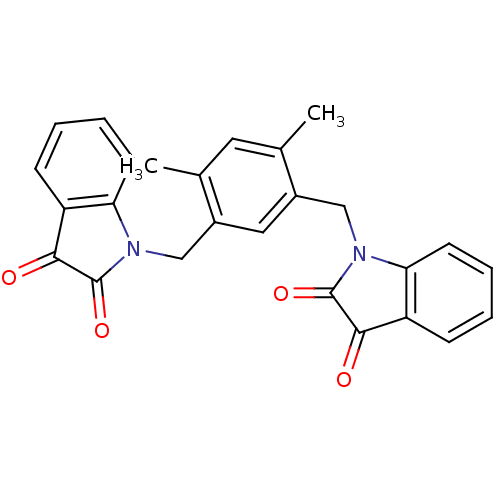
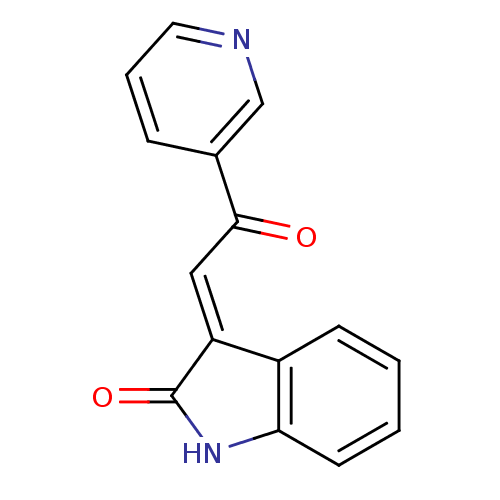
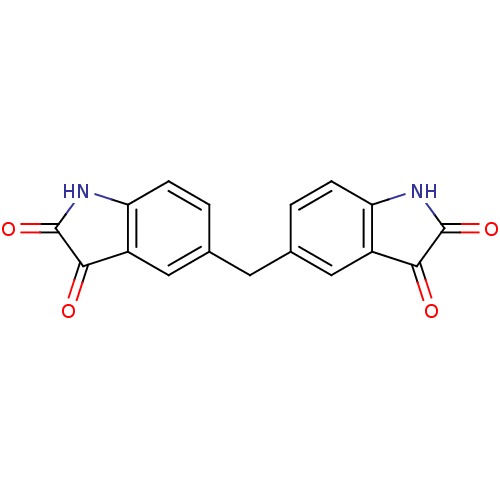
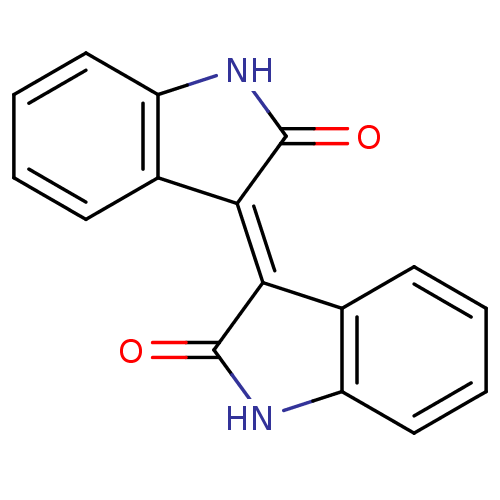
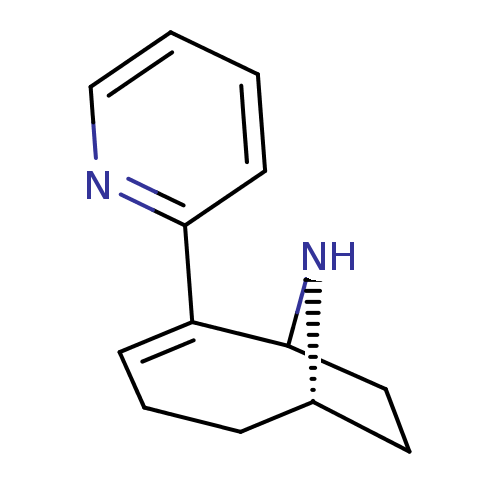
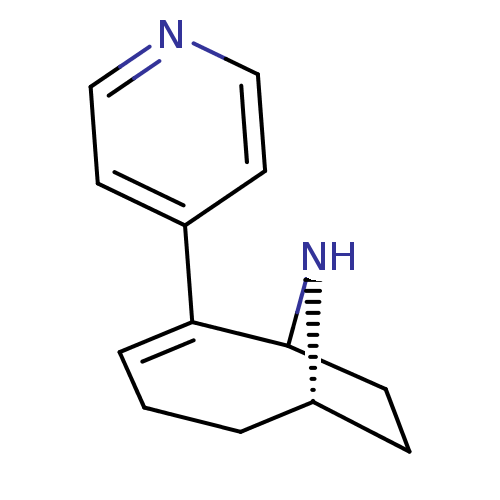
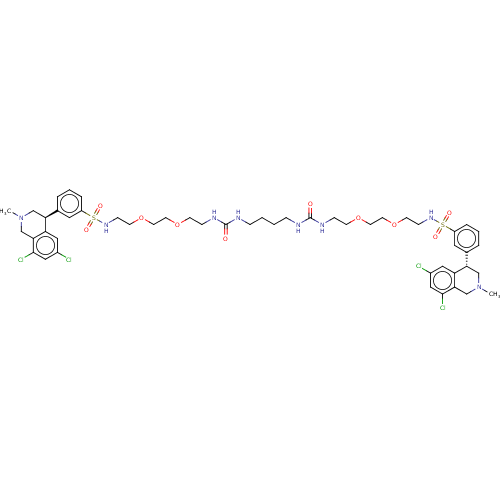
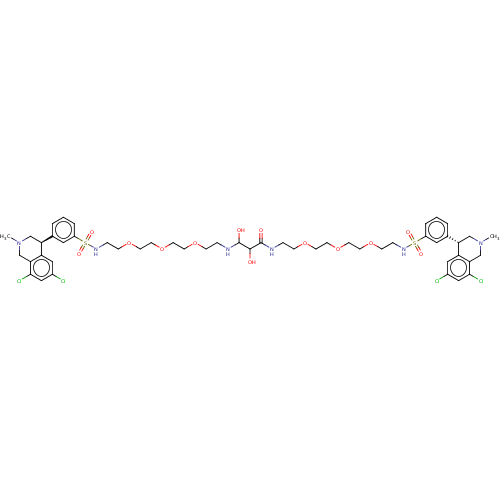
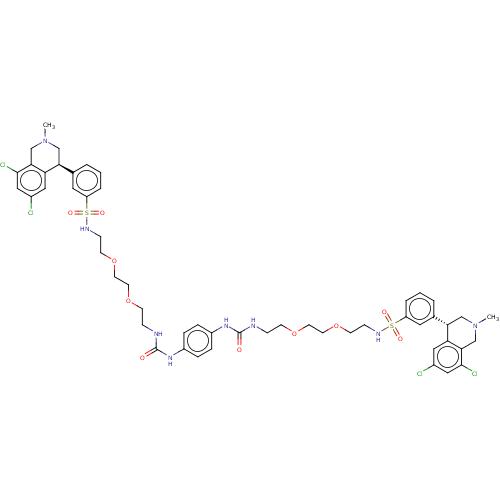
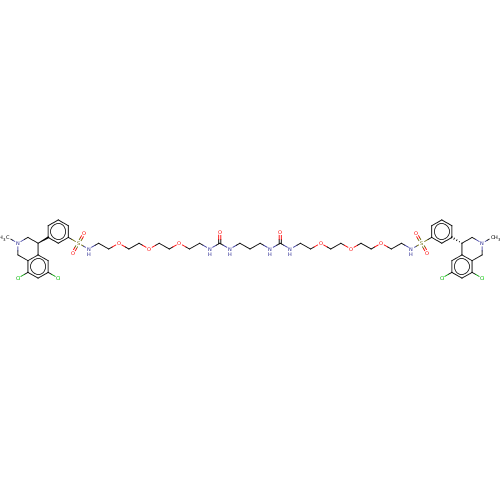
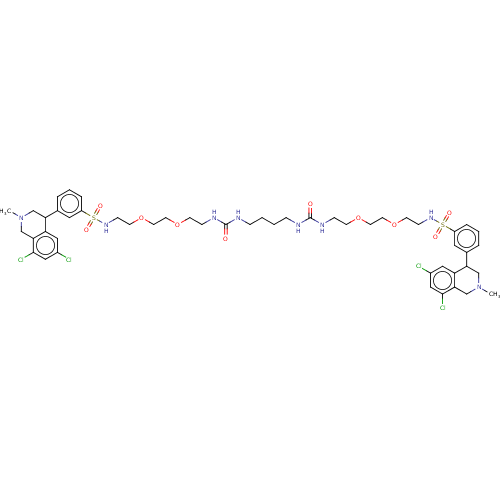
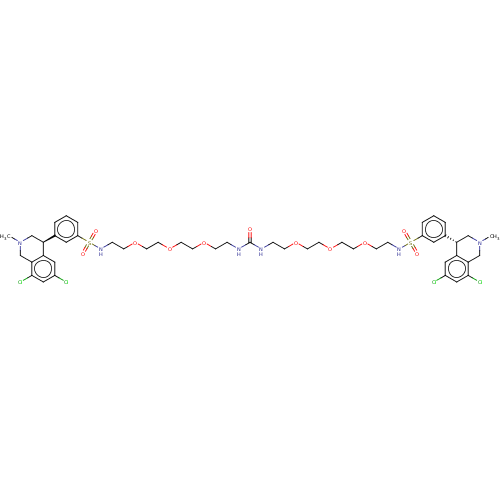
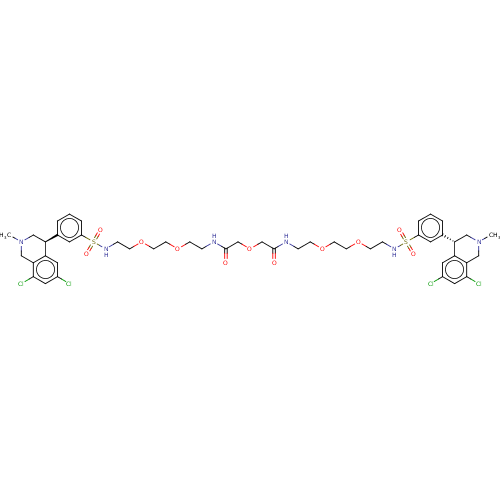
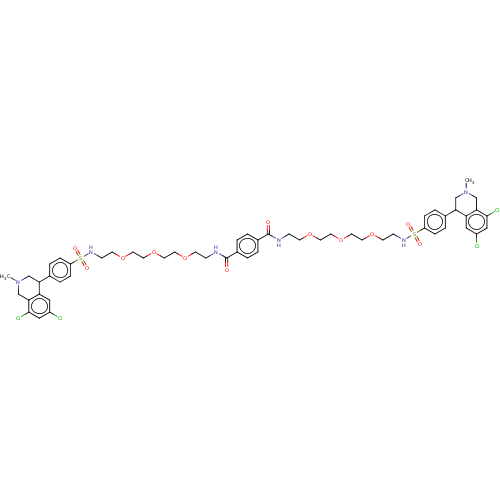
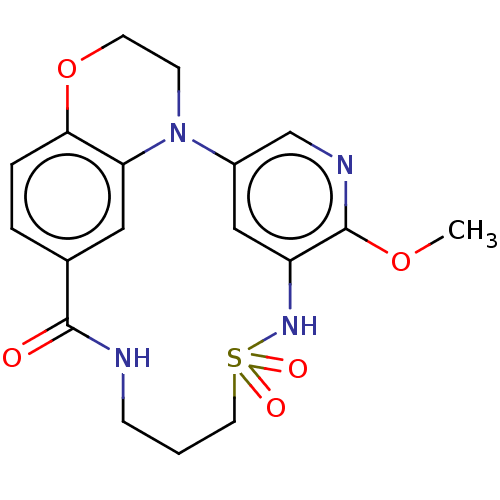
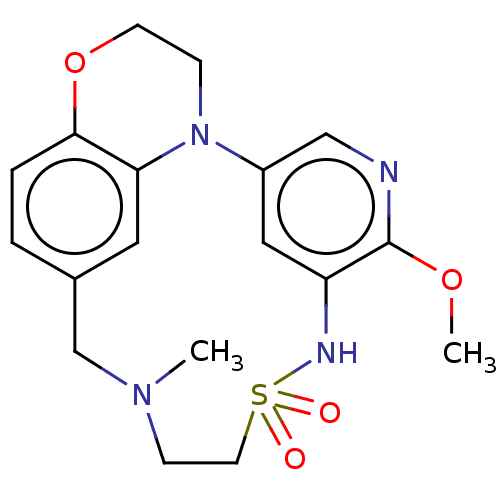
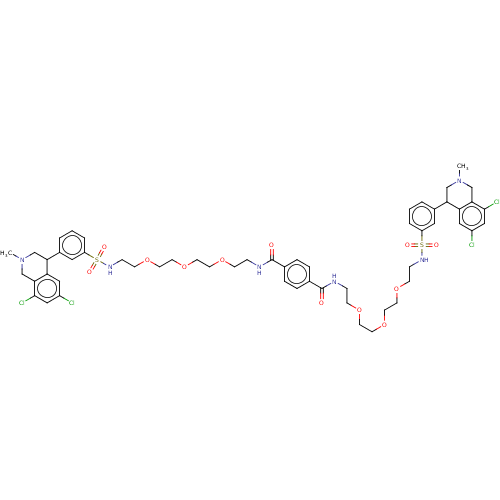
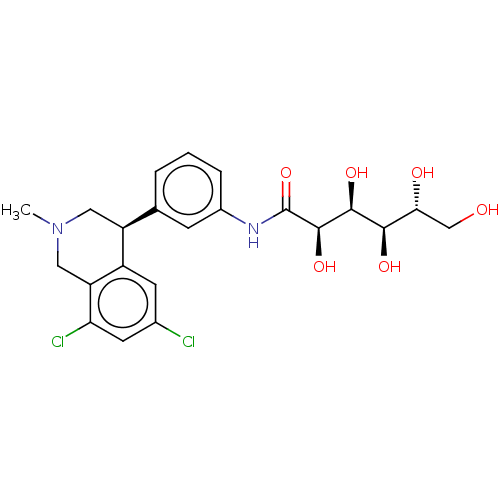
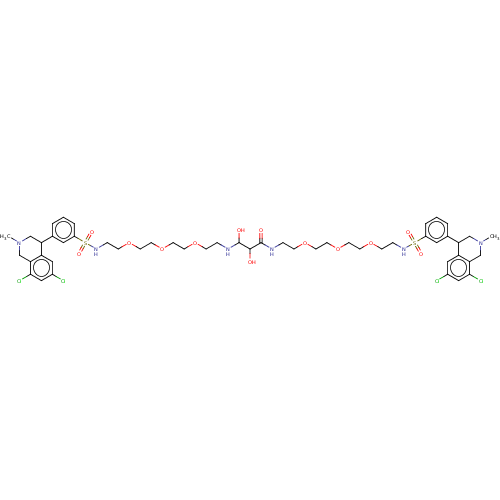
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
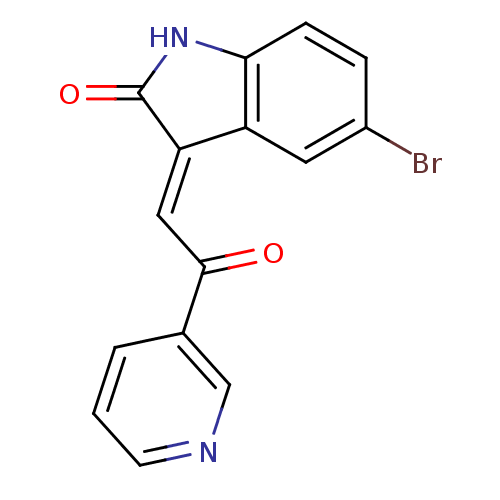
Affinity DataKi: 68.4nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
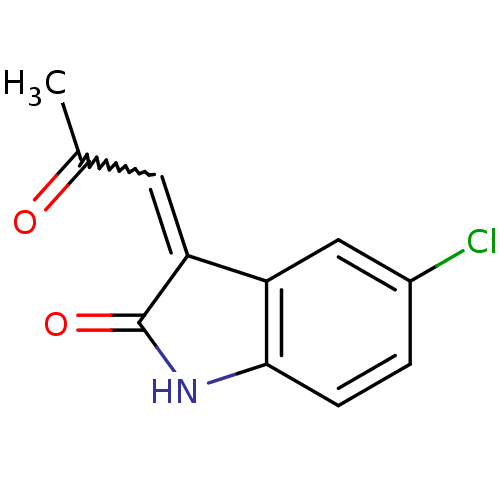
Affinity DataKi: 89nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
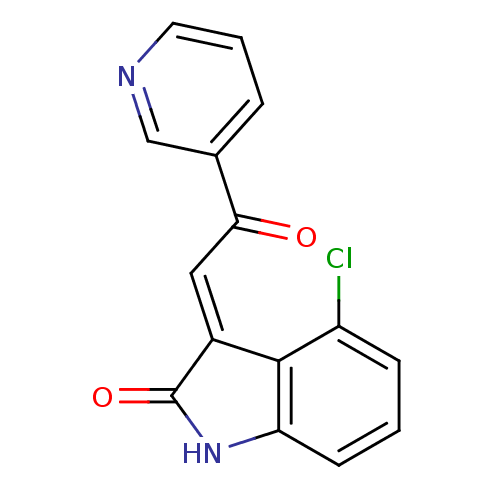
Affinity DataKi: 101nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
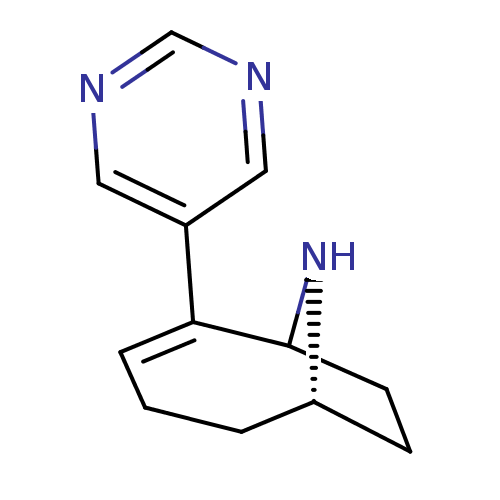
Affinity DataKi: 250nMAssay Description:Inhibition of human transglutaminase 2 using ZGBC as a substrate after 30 to 60 mins by fluorometric assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 410nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 680nMAssay Description:Inhibition of human transglutaminase 2 using ZGBC as a substrate after 30 to 60 mins by fluorometric assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human transglutaminase 2 using ZGBC as a substrate after 30 to 60 mins by fluorometric assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataKi: 1.71E+3nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataKi: 2.76E+3nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 5.30E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 5.40E+3nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataKi: 5.77E+3nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 4.10E+4nMAssay Description:Inhibition of human transglutaminase 2 using Cbz-Gln-Gly as a substrate by GDH-coupled assayMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataKi: 4.75E+4nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataKi: >5.00E+4nMAssay Description:Binding affinity at rat brain Nicotinic acetylcholine receptor alpha7More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
GSK Medicines Research Centre
Curated by ChEMBL
GSK Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
GSK Medicines Research Centre
Curated by ChEMBL
GSK Medicines Research Centre
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)