TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
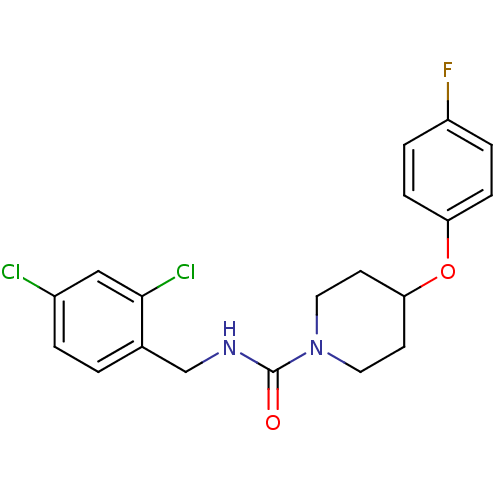
Affinity DataIC50: 0.100nMAssay Description:Inhibition of soluble EH in human HepG2 cells by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
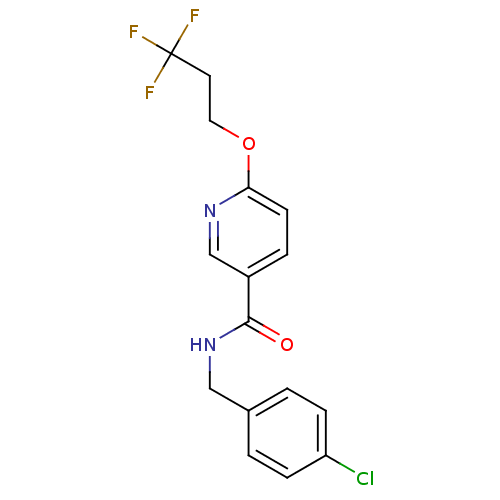
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
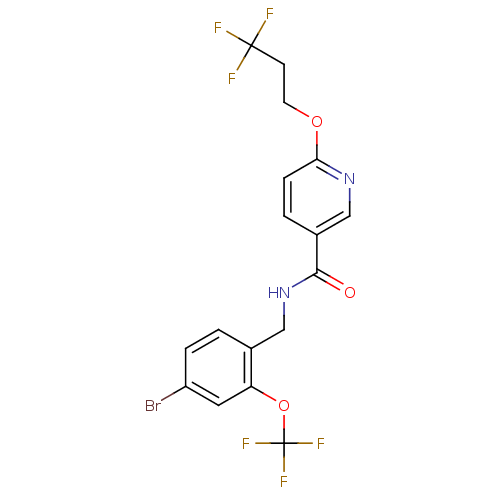
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human soluble EHMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human soluble epoxide hydrolase assessed as [2-3H]-trans-1,3-diphenyl propylene oxide hydrolysis by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Inhibition of rat sEHMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human soluble EHMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of NHE1 in human HT-29 cells assessed as intracellular pH change after 30 minsMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Inhibition of human soluble epoxide hydrolase in human HepG cells assessed as conversion of epoxyeicosatienoic acid to dihydroxyeicosatrienoic acid a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.40nMAssay Description:Inhibition of human soluble EHMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.90nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of [125I]-U2 from human recombinant urotensin2 receptor expressed in human Chem-2 cells after 4 hrs by scintillation proximity assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSodium/hydrogen exchanger 1(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of NHE1 in human HT-29 cells assessed as intracellular pH change after 30 minsMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6.40nMAssay Description:Displacement of rhodamine-labeled probe from human soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 6.60nMAssay Description:Inhibition of human soluble EHMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of human soluble EHMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
TargetBifunctional epoxide hydrolase 2(Rattus norvegicus)
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Displacement of rhodamine-labeled probe from rat soluble epoxide hydrolase by fluorescence polarization assayMore data for this Ligand-Target Pair

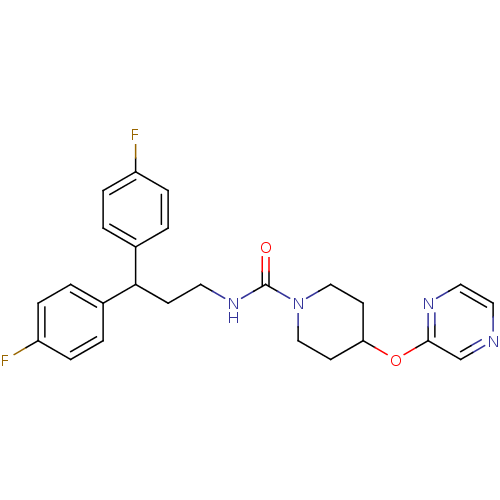
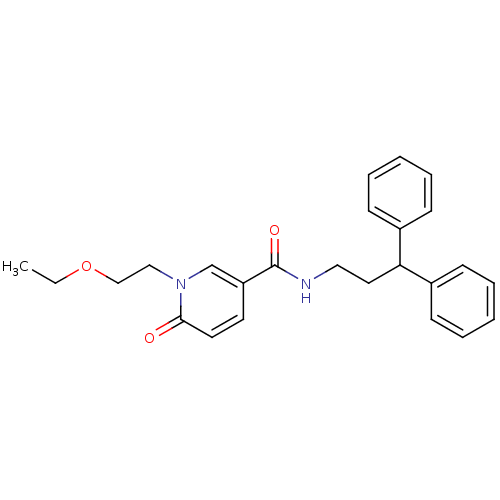
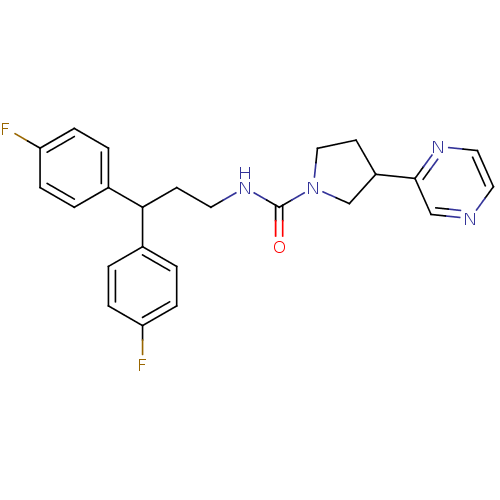

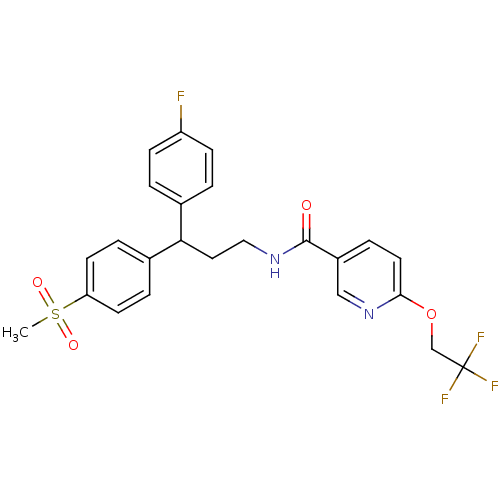
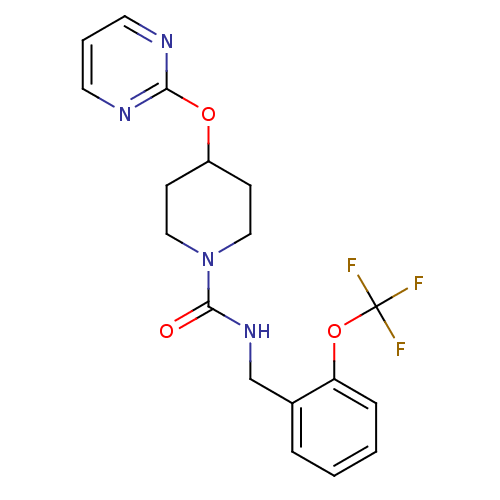
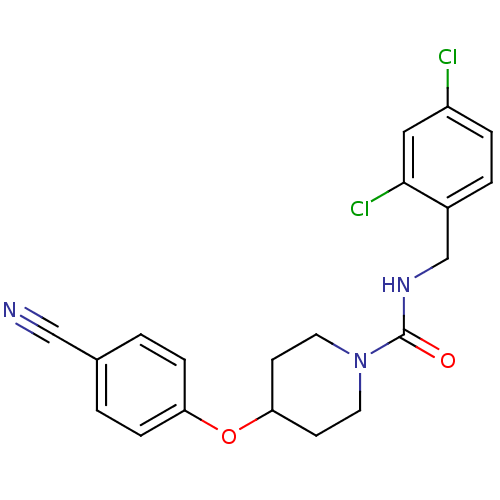
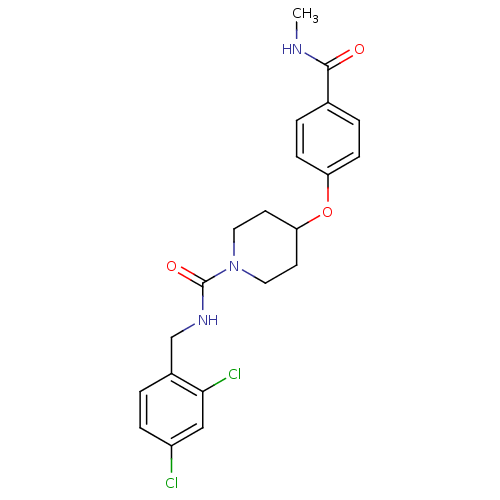
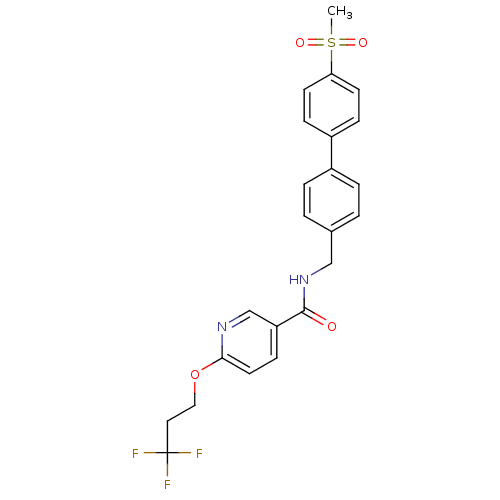
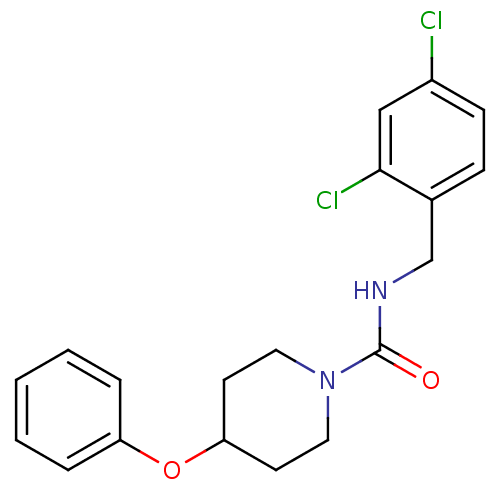

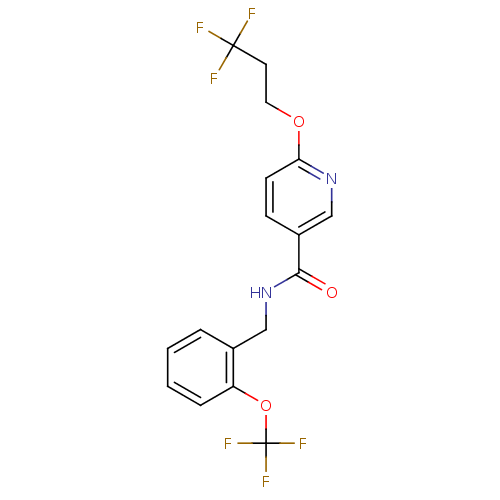
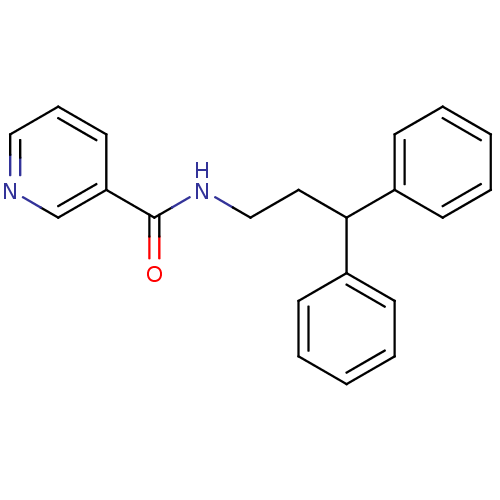
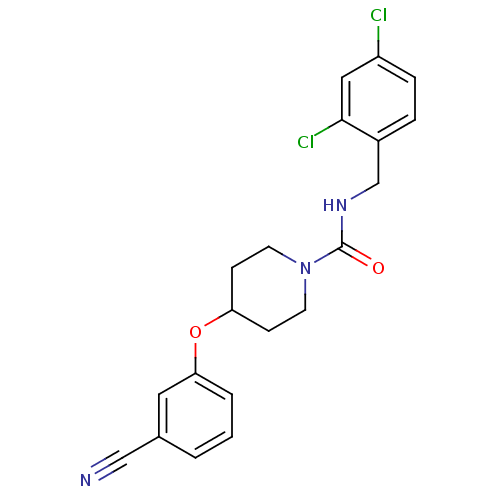

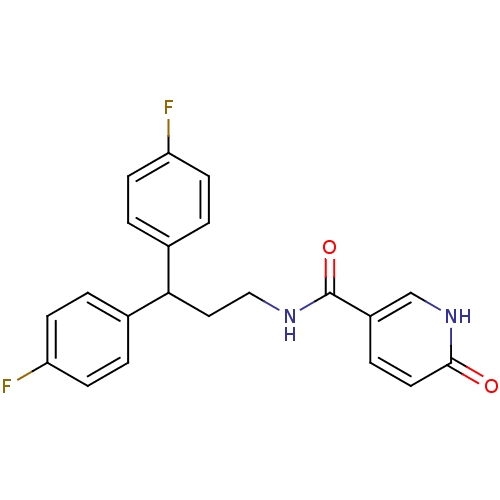
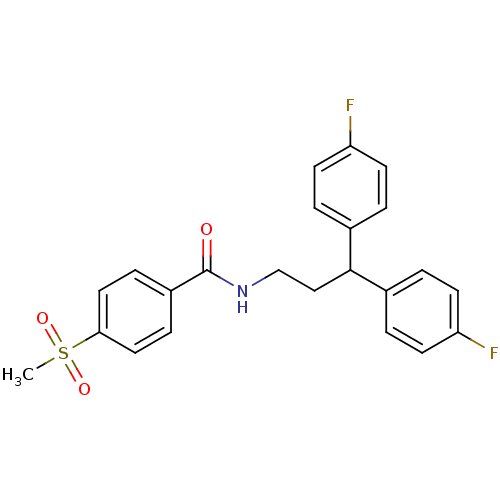
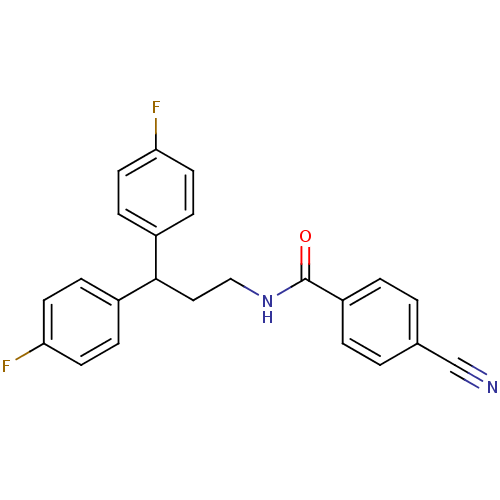
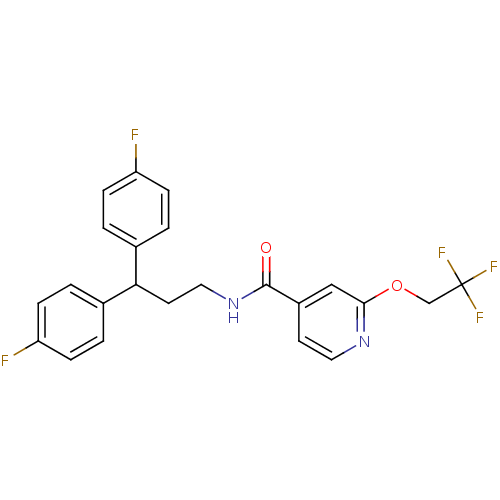
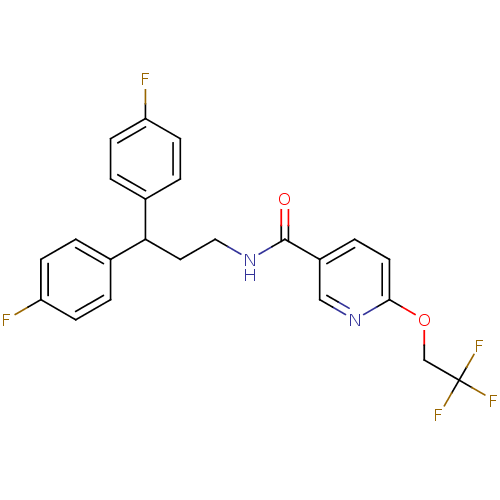
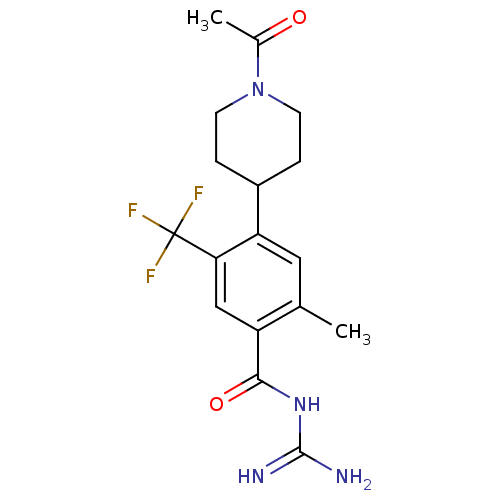
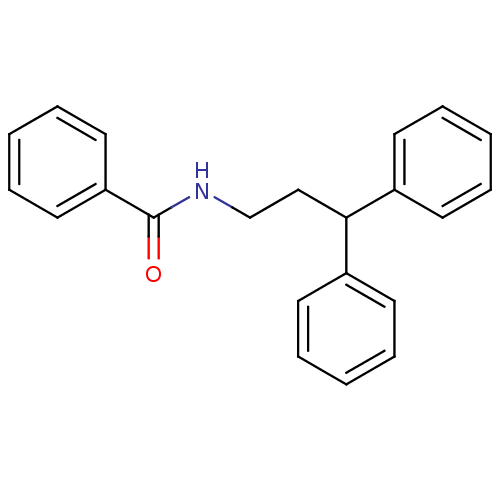
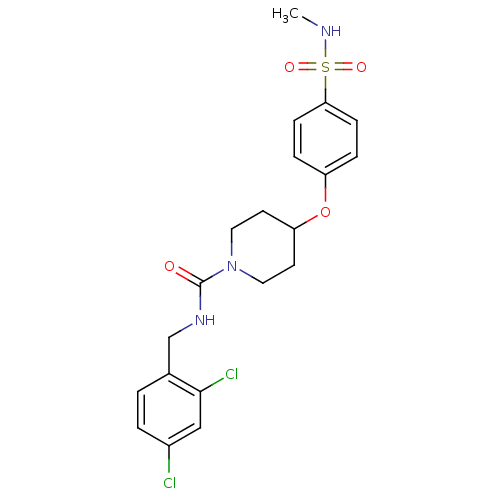
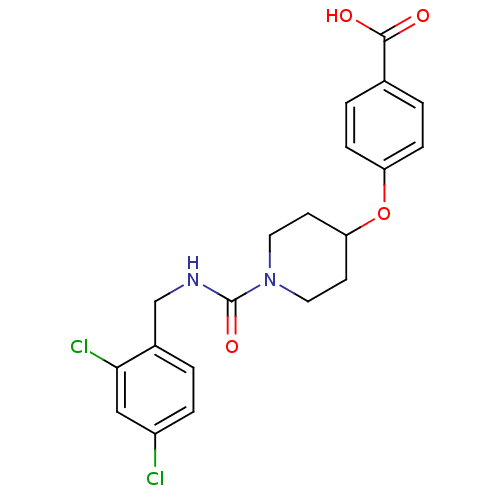

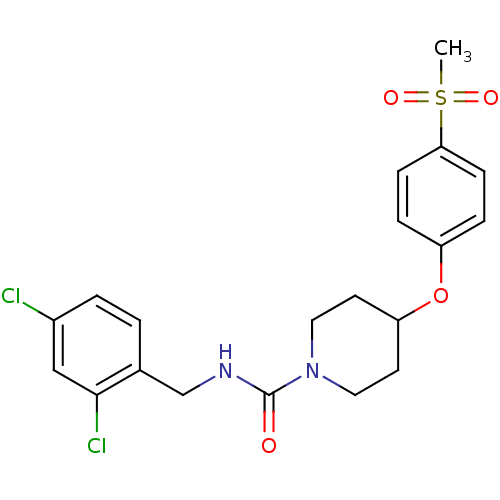
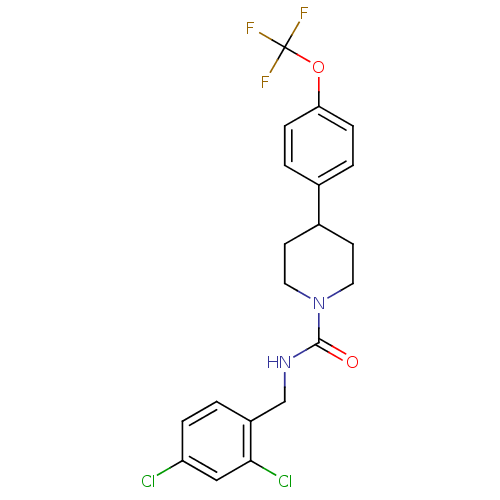
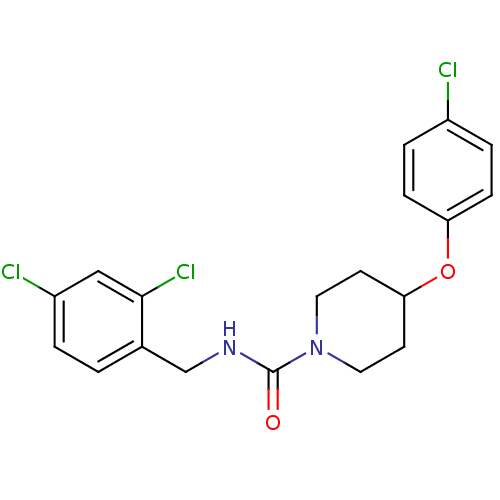

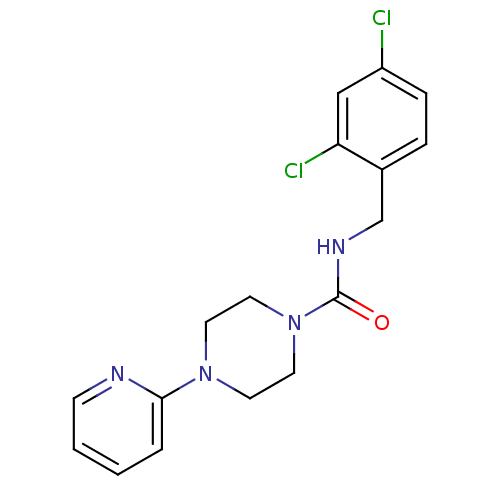
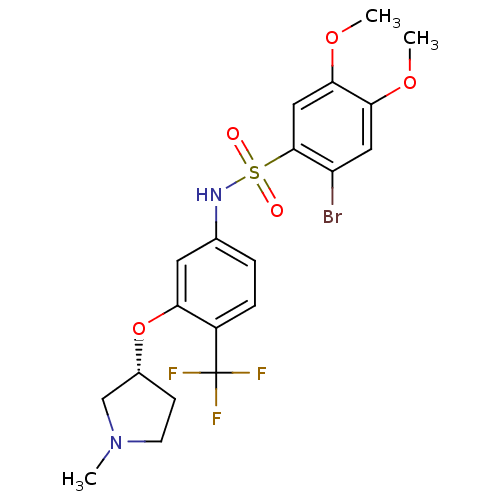
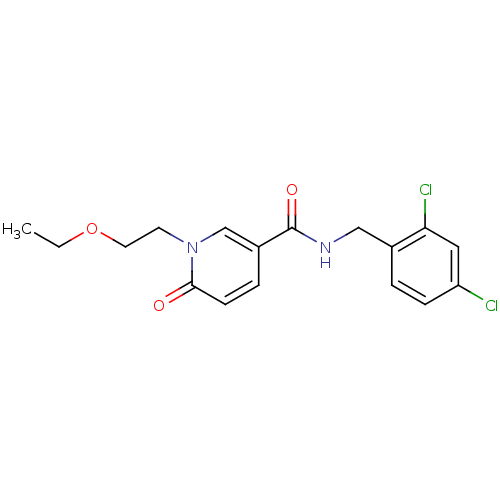
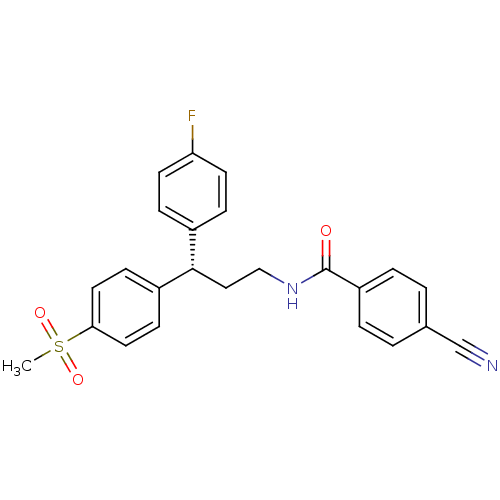
 3D Structure (crystal)
3D Structure (crystal)