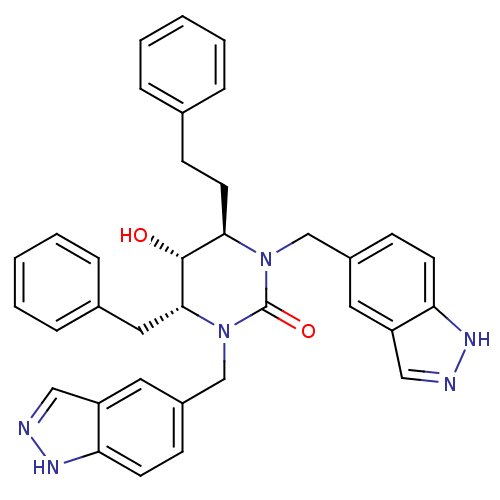
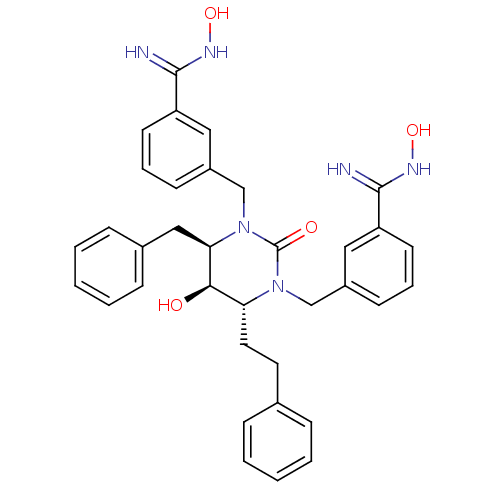
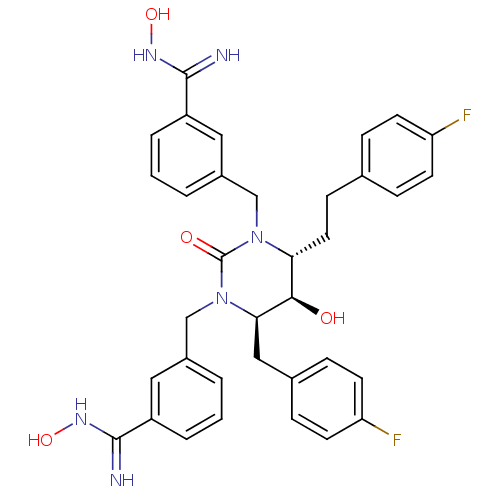
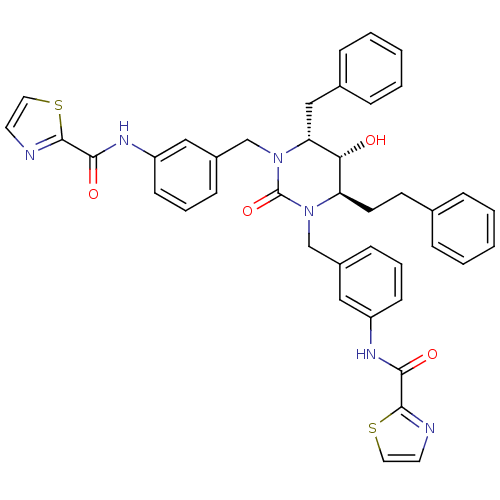
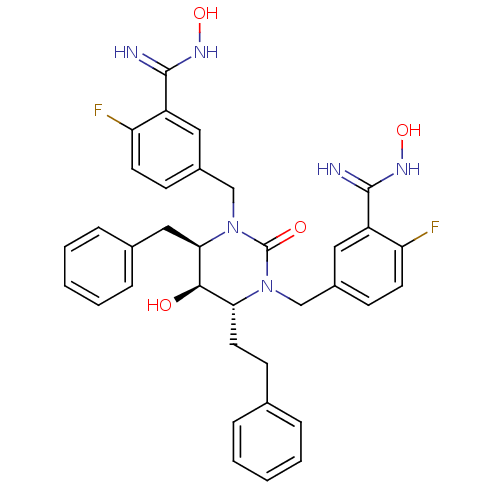
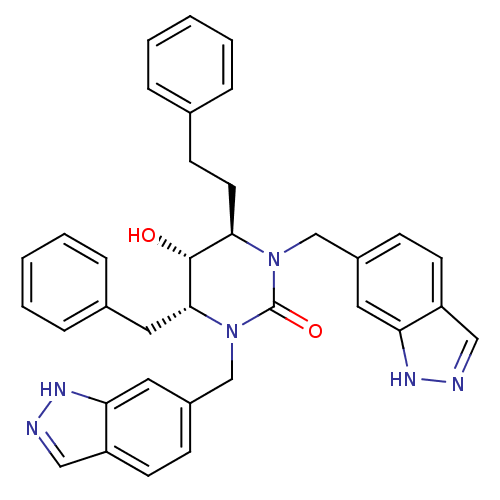
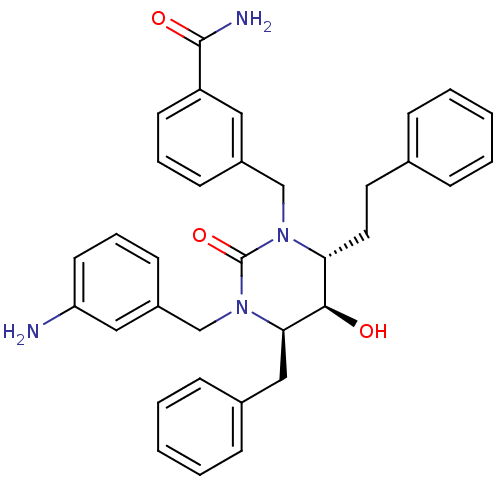
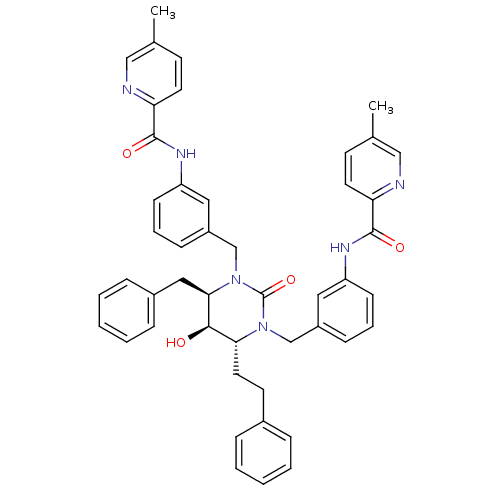
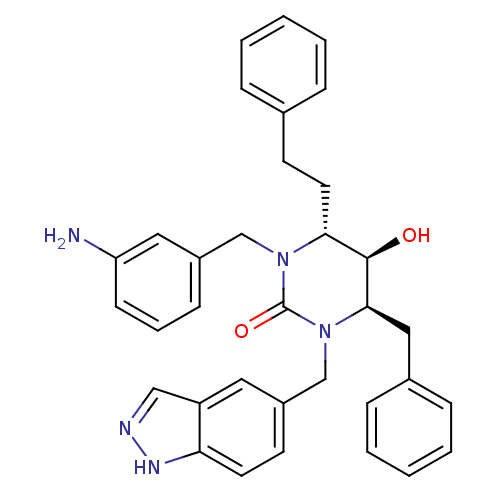
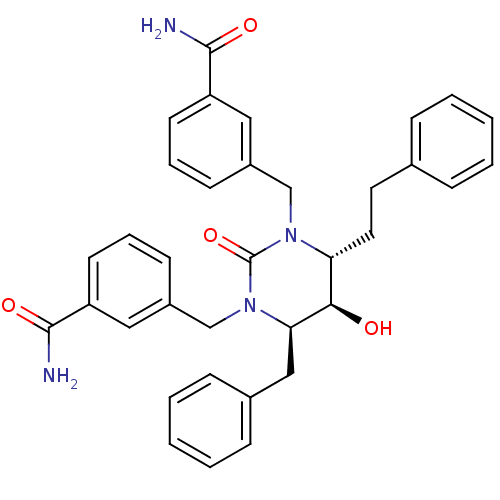
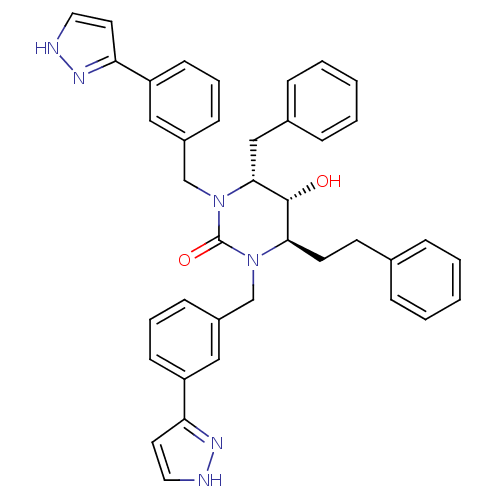
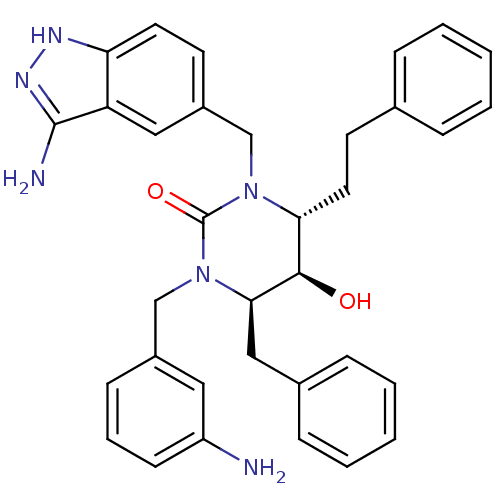
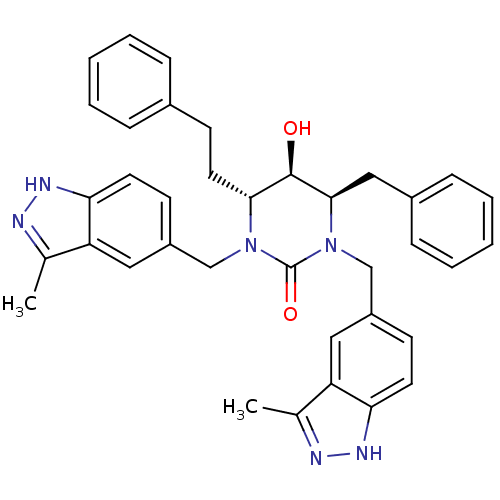
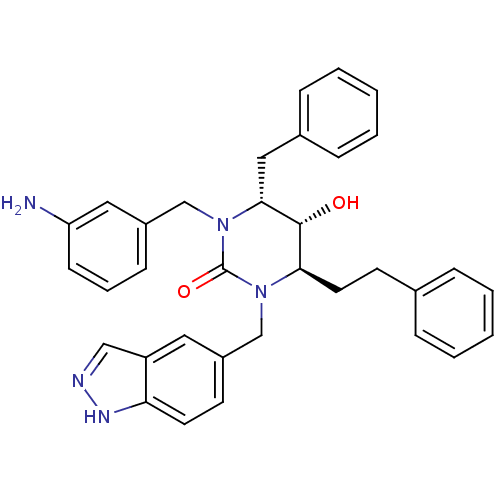
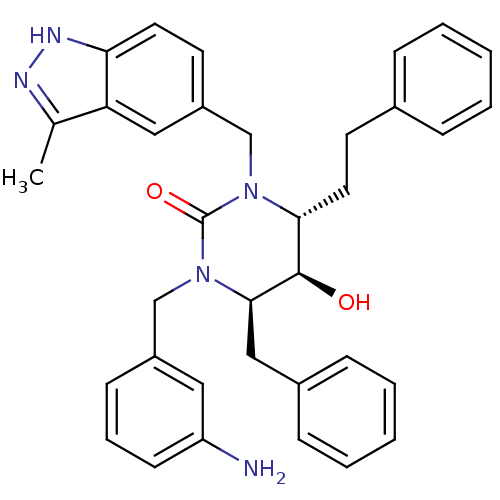
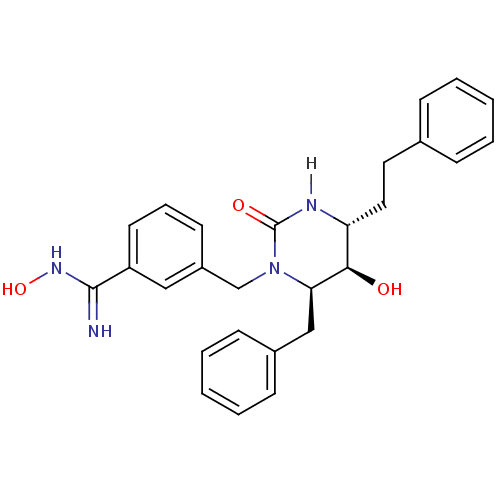
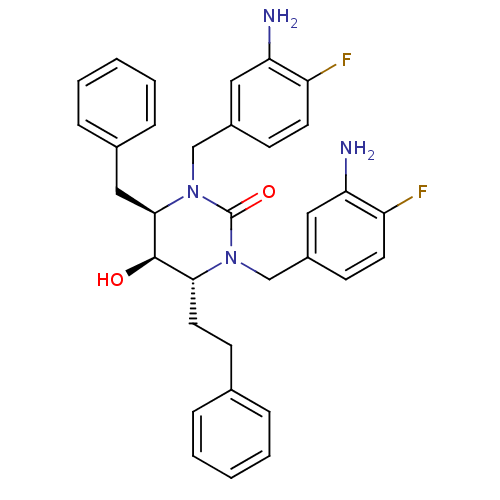
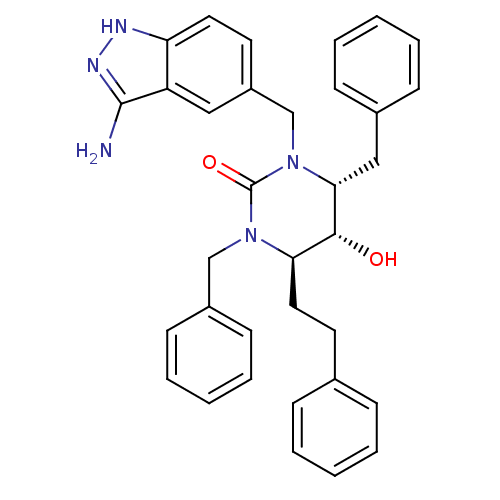
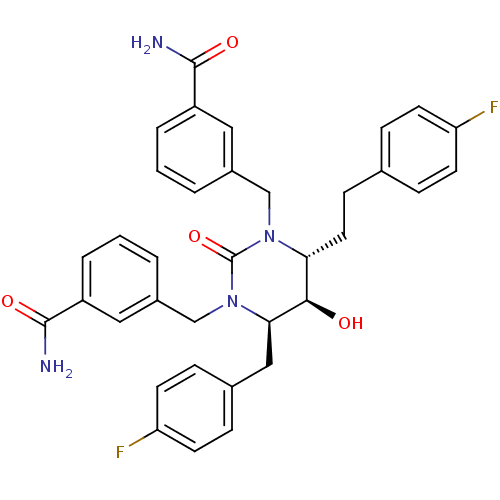
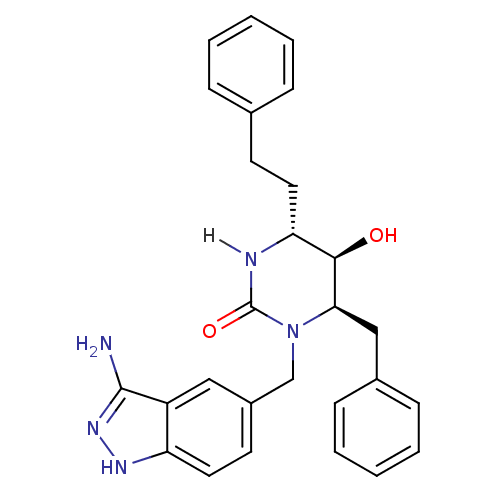
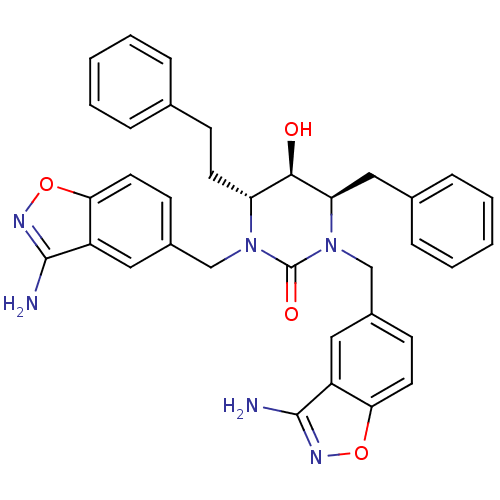
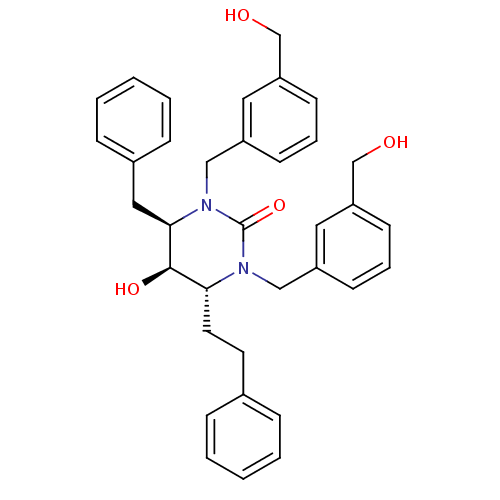
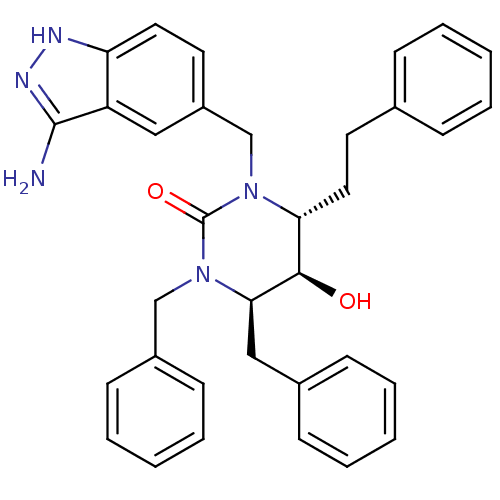
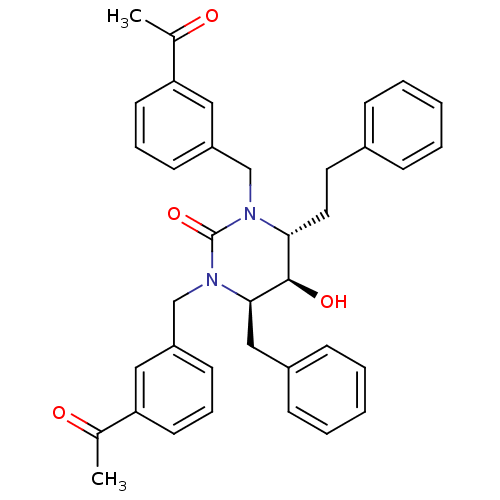
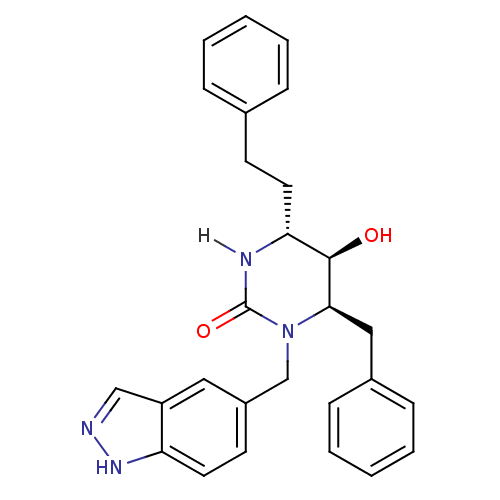
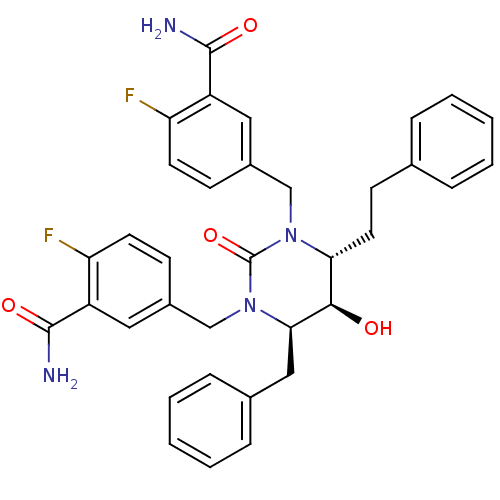
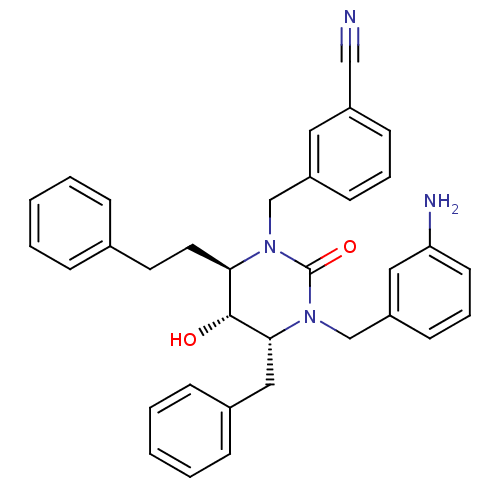
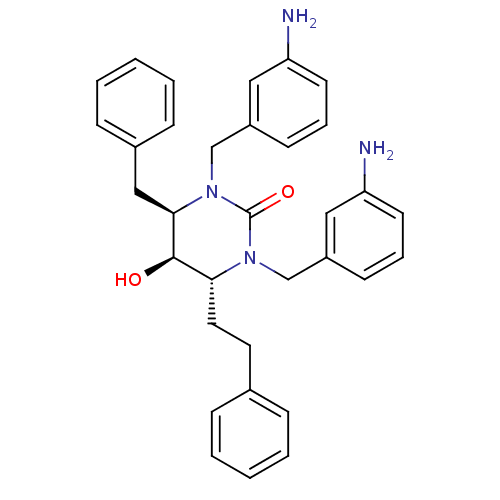
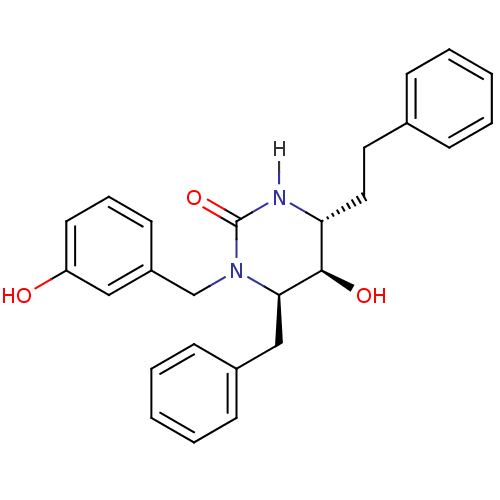
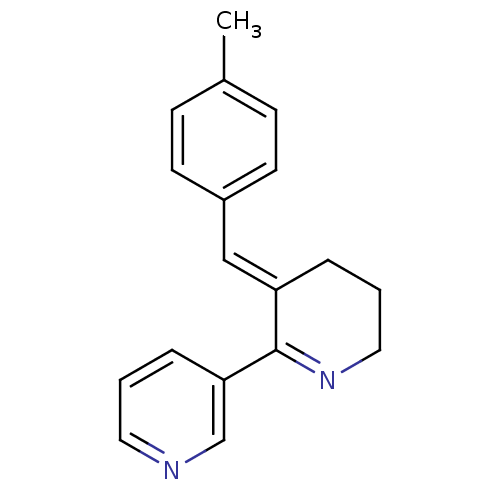
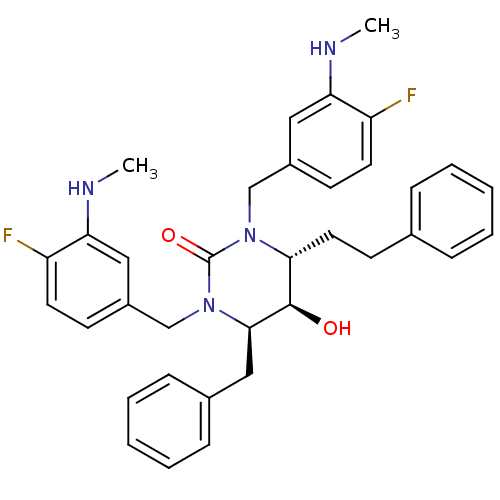
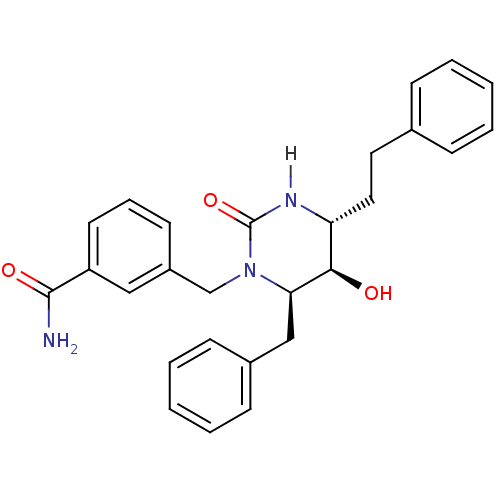
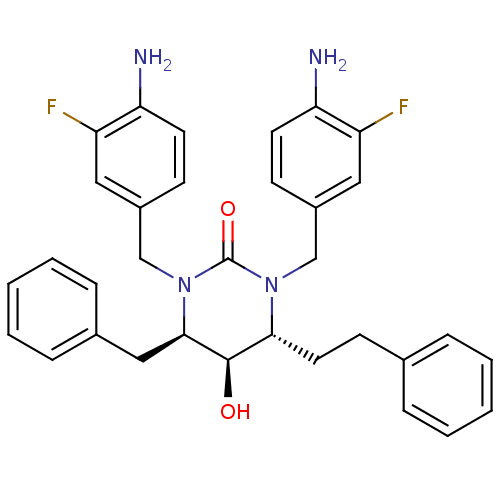
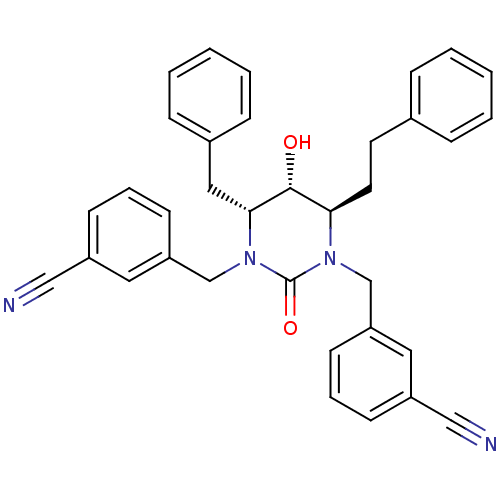
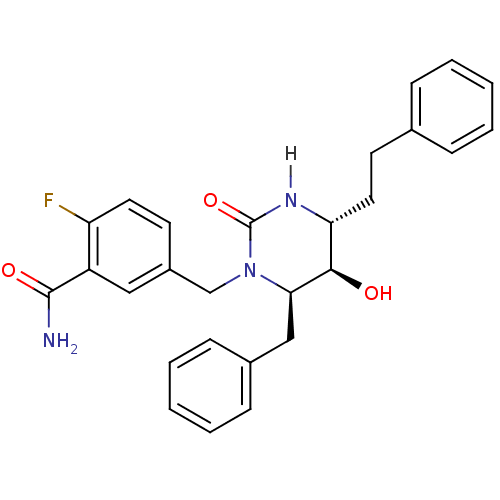
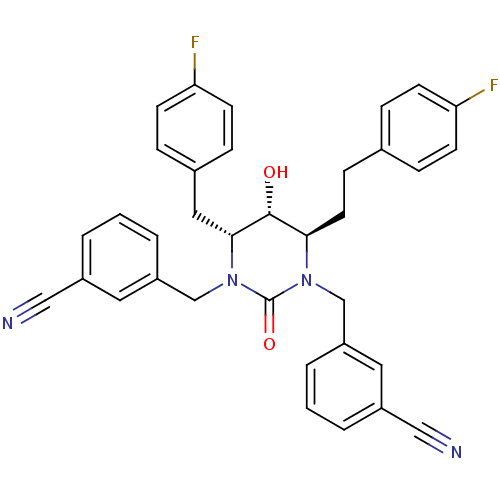
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0100nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0301nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0301nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0601nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0601nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0601nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0802nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0802nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.0902nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.150nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.240nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.250nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.281nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.310nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.410nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.481nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.491nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.871nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 0.912nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 4.90nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 5.60nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 6.20nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibitory activity of compound against HIV-1 aspartyl protease.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Rattus norvegicus (Rat))
University of Florida
Curated by ChEMBL
University of Florida
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane after 3 hrsMore data for this Ligand-Target Pair