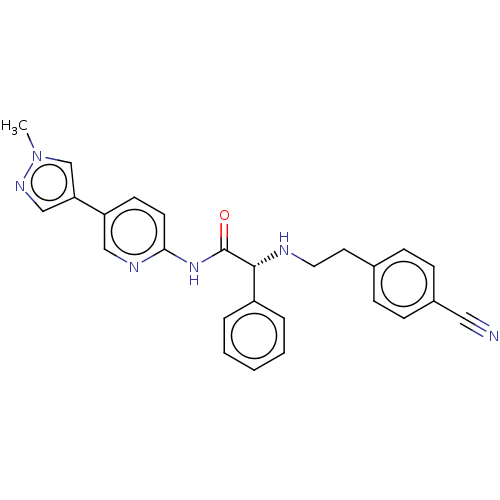
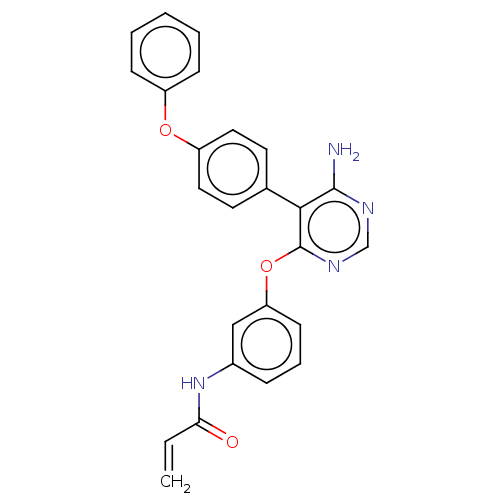
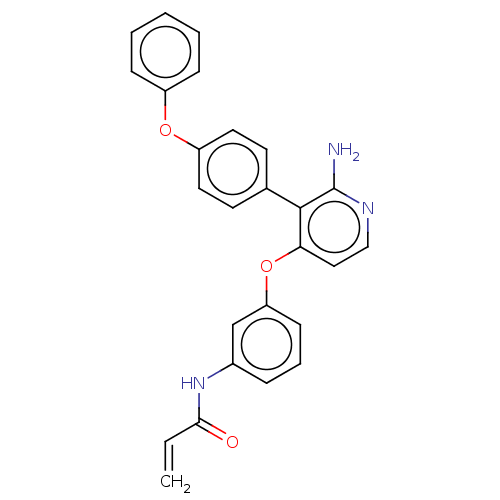
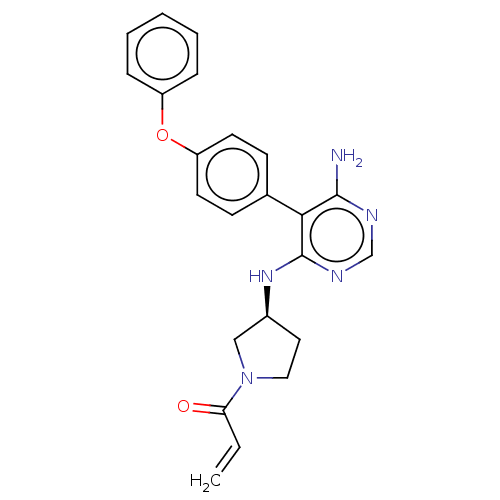
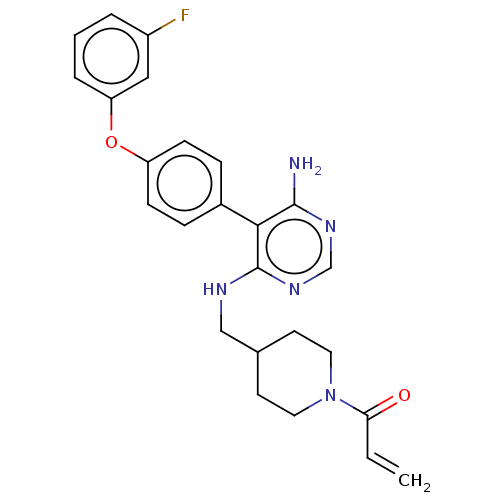
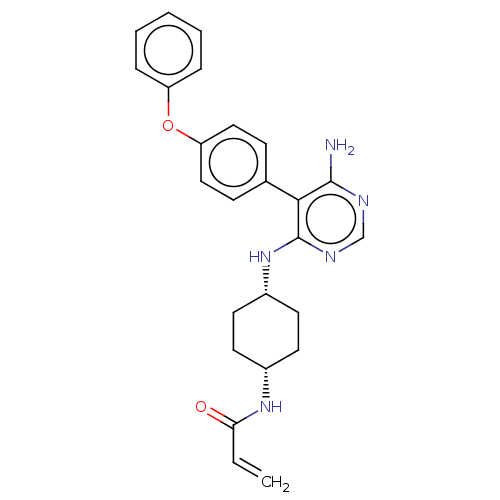
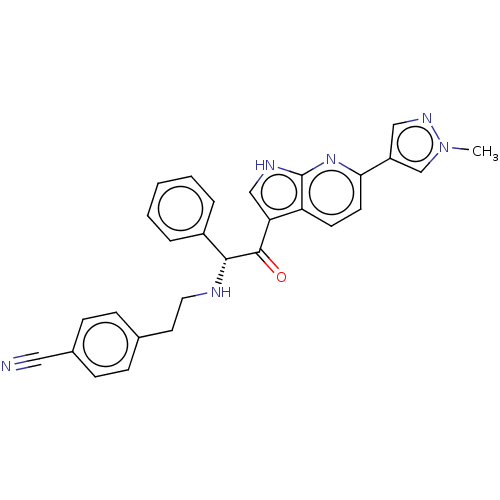
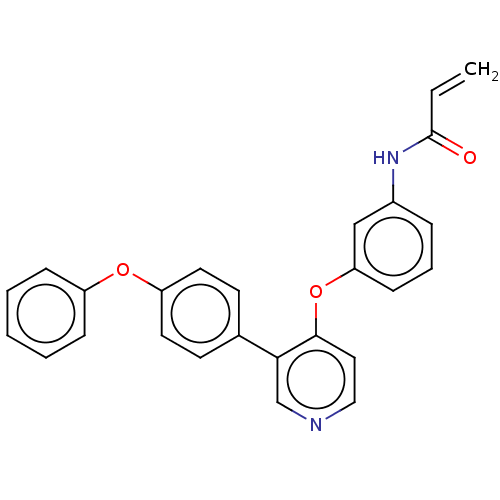
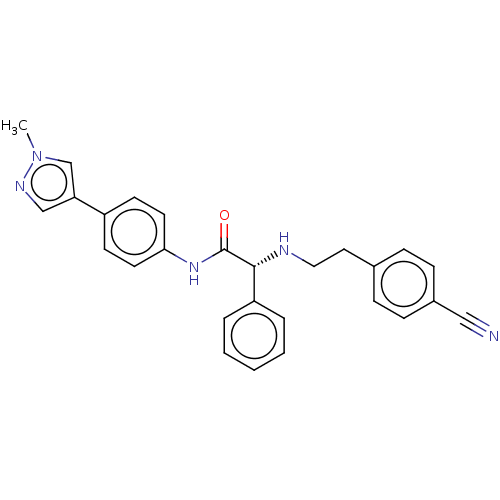
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck KGaA
Curated by ChEMBL
Merck KGaA
Curated by ChEMBL
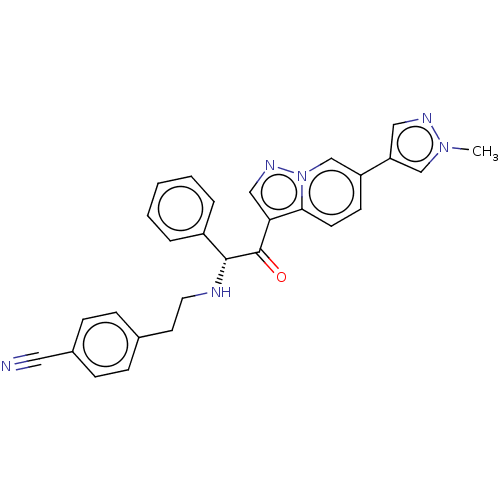
Affinity DataKi: 530nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
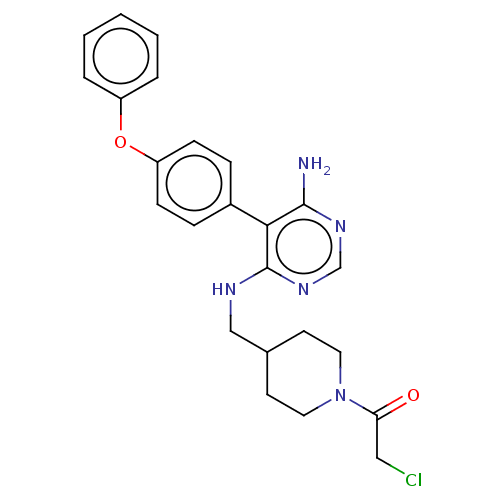
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck KGaA
Curated by ChEMBL
Merck KGaA
Curated by ChEMBL
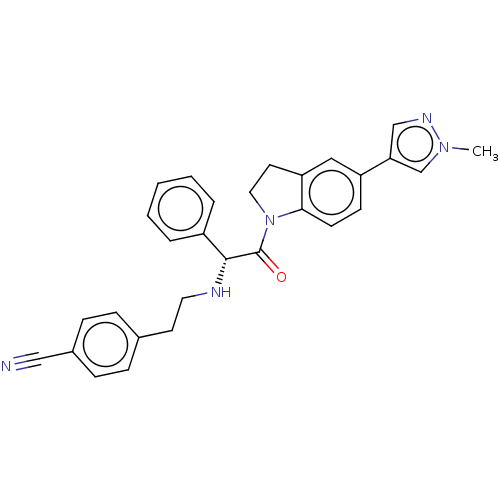
Affinity DataKi: 780nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
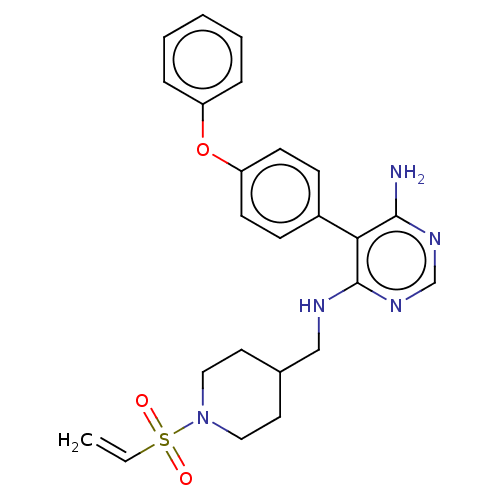
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck KGaA
Curated by ChEMBL
Merck KGaA
Curated by ChEMBL
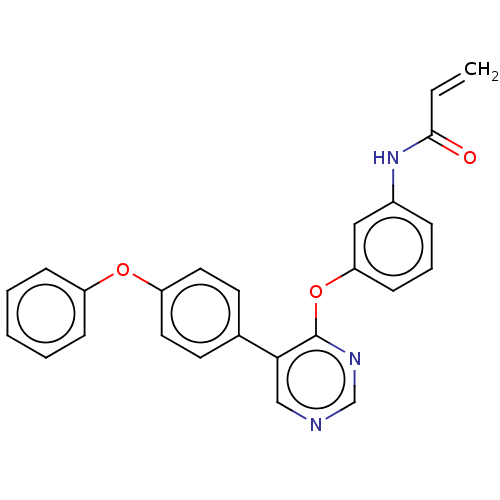
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
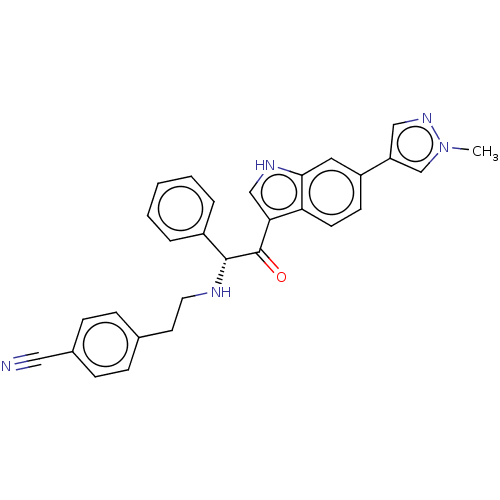
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck KGaA
Curated by ChEMBL
Merck KGaA
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck KGaA
Curated by ChEMBL
Merck KGaA
Curated by ChEMBL
Affinity DataKi: 3.10E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
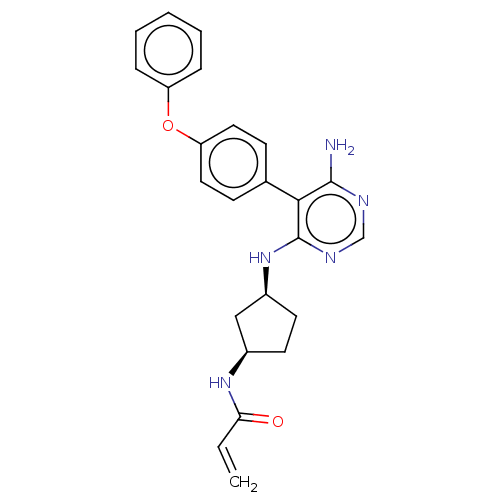
Affinity DataIC50: 0.5nMAssay Description:Binding affinity to recombinant full length EP300 (unknown origin) incubated for 30 mins followed by H3(1 to 21) addition and measured after 1 hr by ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Binding affinity to recombinant full length EP300 (unknown origin) incubated for 30 mins followed by H3(1 to 21) addition and measured after 1 hr by ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Binding affinity to recombinant full length EP300 (unknown origin) incubated for 30 mins followed by H3(1 to 21) addition and measured after 1 hr by ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Binding affinity to recombinant full length CBP (unknown origin) incubated for 30 mins followed by H3(1 to 21) addition and measured after 1 hr by sc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Binding affinity to recombinant full length CBP (unknown origin) incubated for 30 mins followed by H3(1 to 21) addition and measured after 1 hr by sc...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Binding affinity to recombinant full length CBP (unknown origin) incubated for 30 mins followed by H3(1 to 21) addition and measured after 1 hr by sc...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 8.10nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 9.90nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 61nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 66nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go...More data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cellsMore data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair
TargetHistone acetyltransferase p300(Homo sapiens (Human))
Constellation Pharmaceuticals
Curated by ChEMBL
Constellation Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Binding affinity to recombinant N-terminal His-tagged EP300 HAT domain (unknown origin) (1287 to 1666 residues) expressed in Escherichia coli incubat...More data for this Ligand-Target Pair

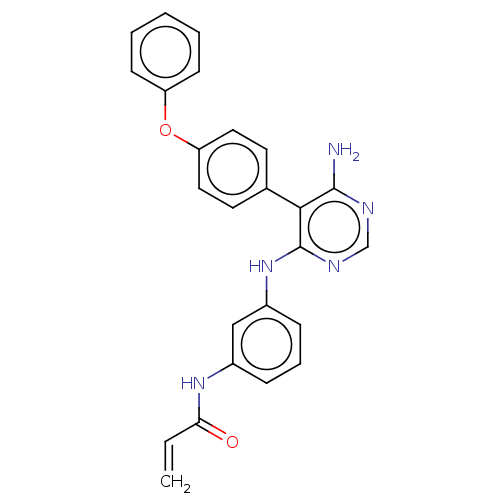


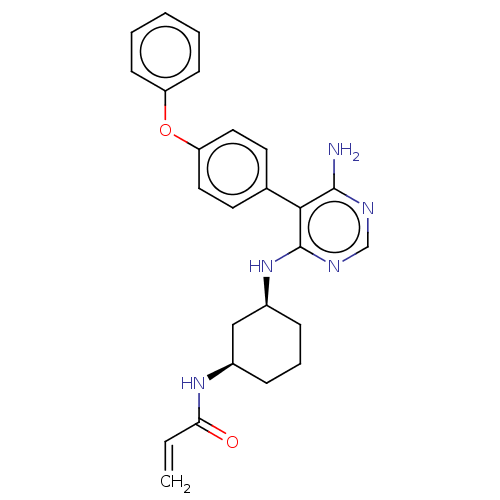

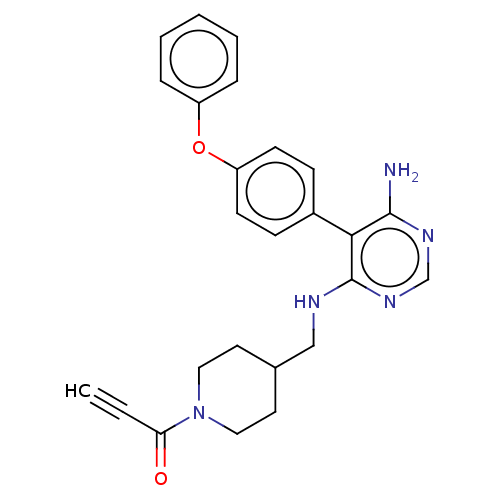
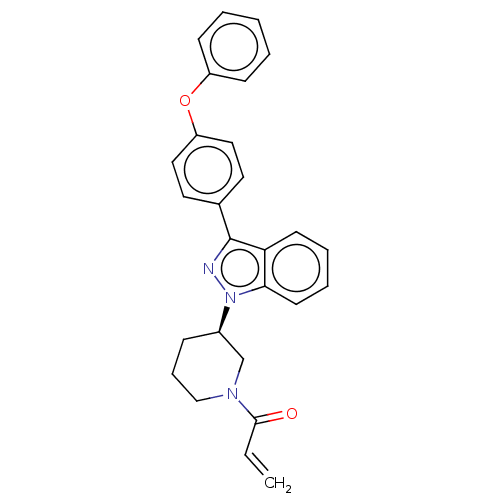
 3D Structure (crystal)
3D Structure (crystal)