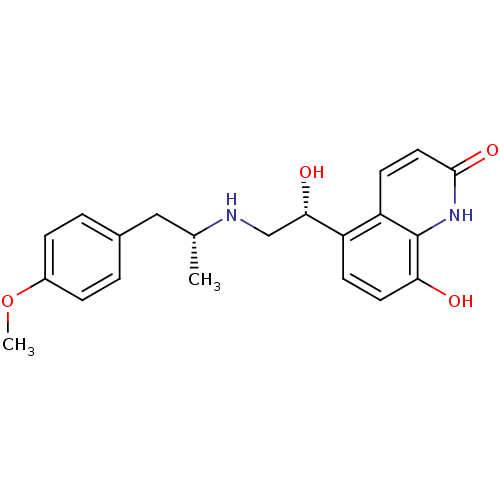
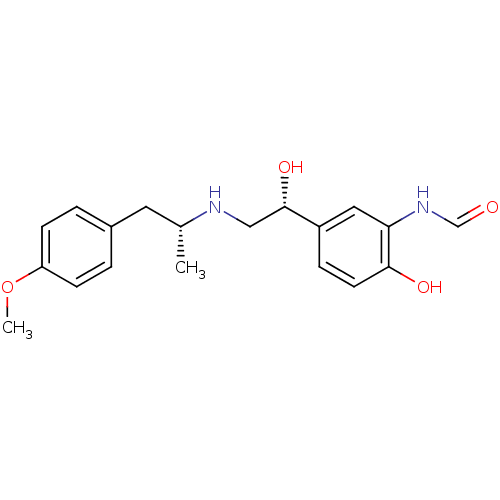
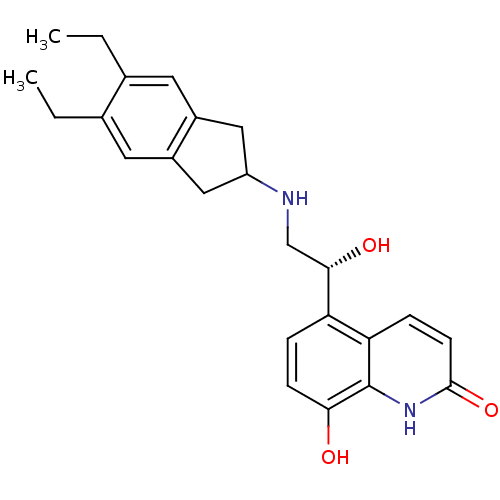
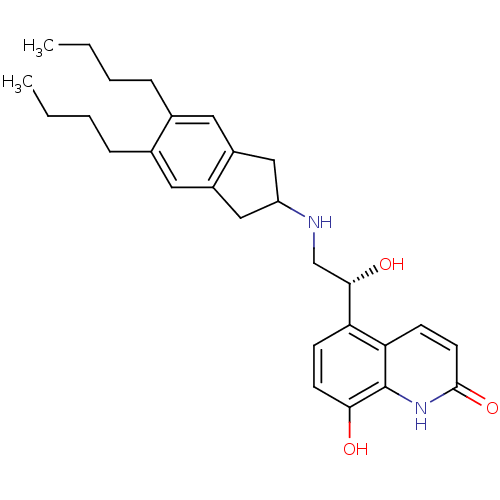
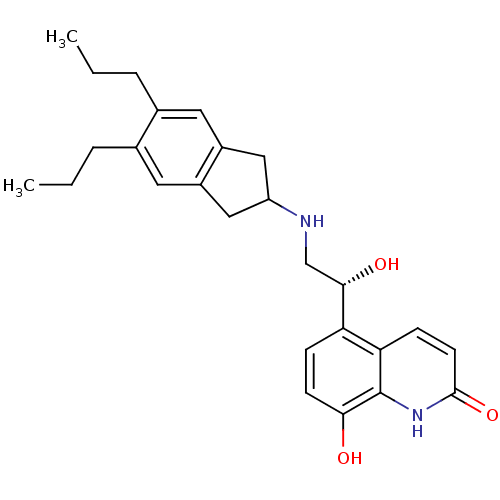
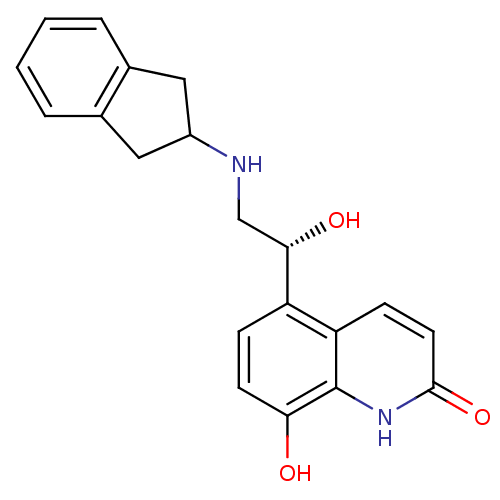
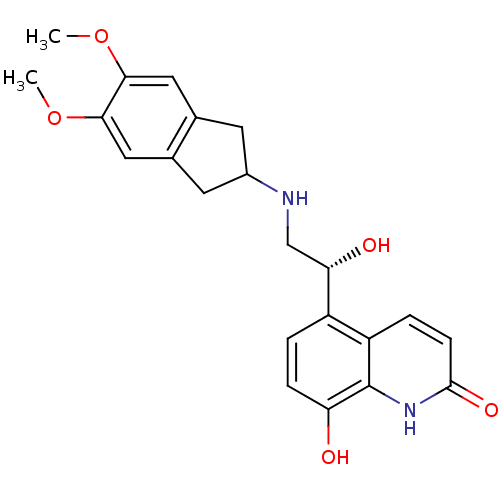
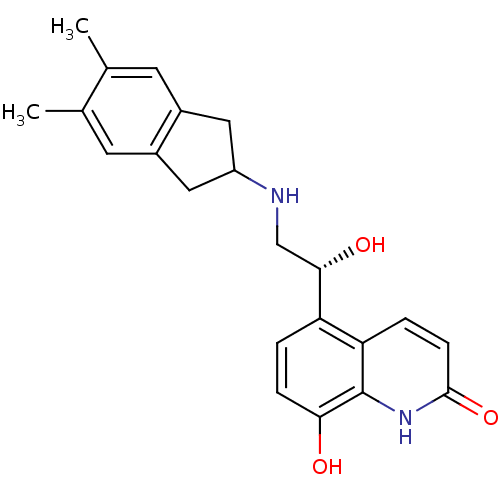
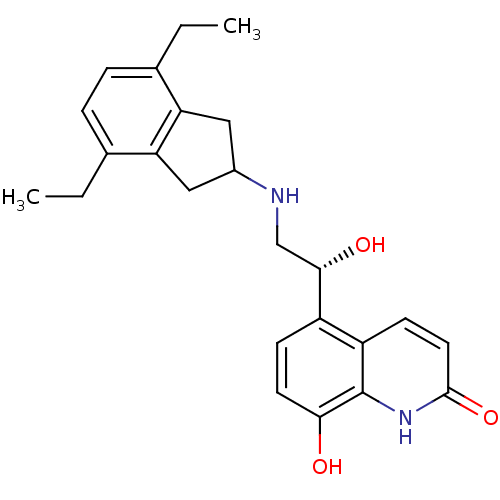
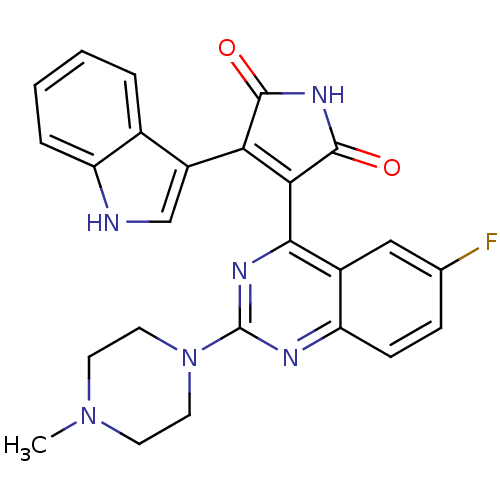
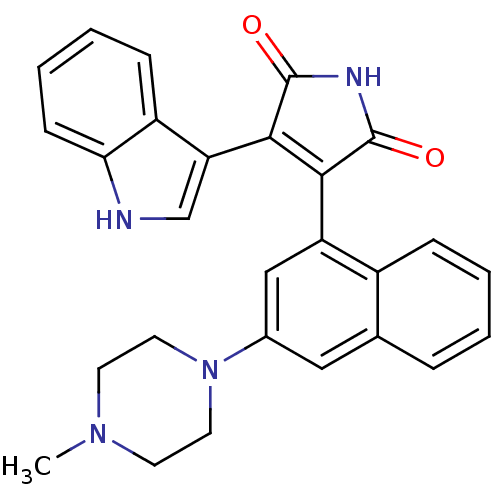
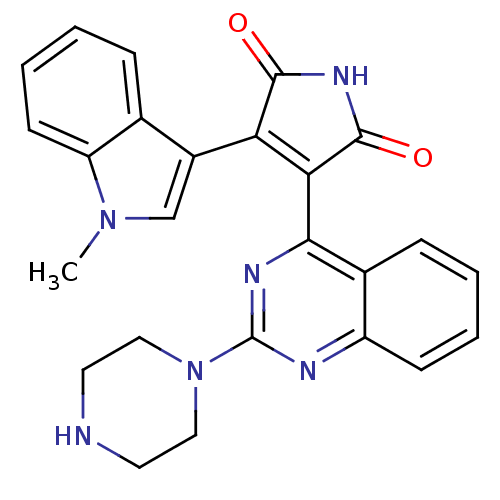
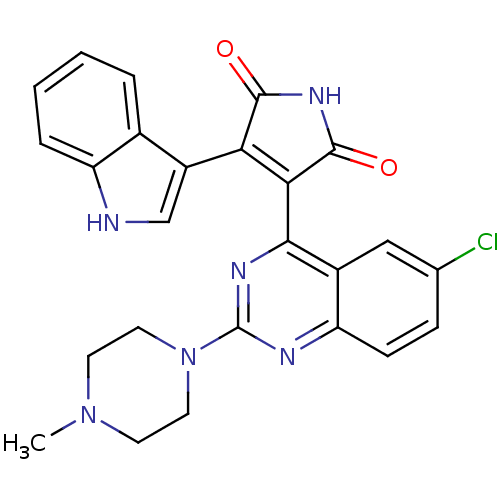
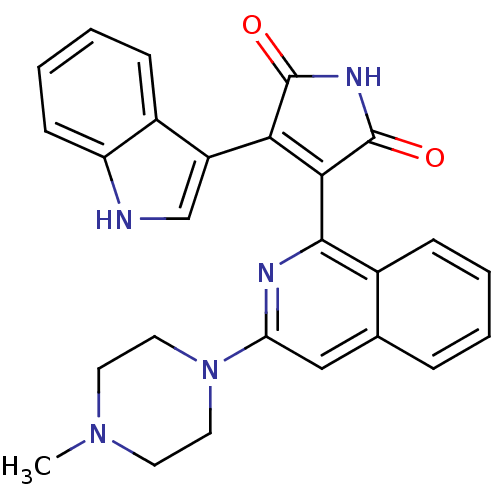
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.390nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
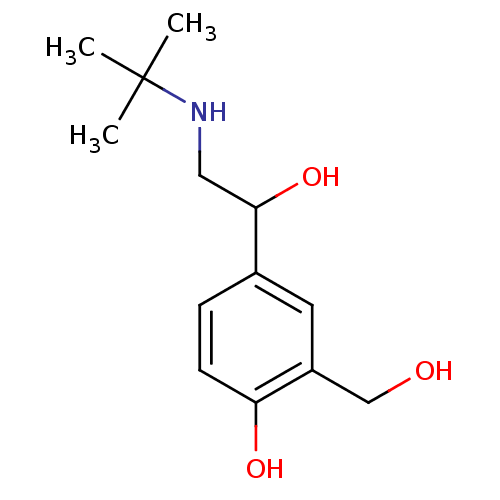
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
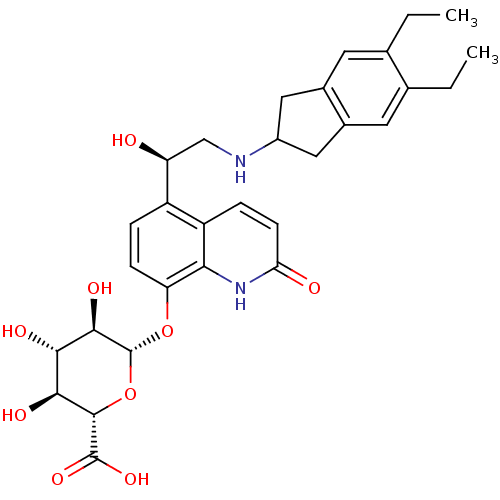
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 23nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
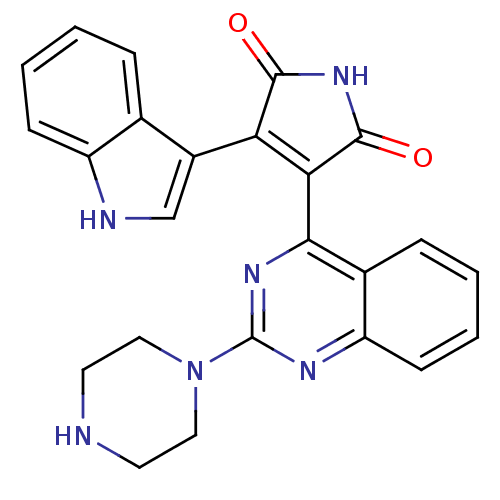
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 76nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 112nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 119nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 218nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 342nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 522nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 692nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: 1.83E+3nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Inhibition of CYP3A4-catalyzed midazolam 1'-hydroxylation in human liver microsomesMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(GUINEA PIG)
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(GUINEA PIG)
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response...More data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C epsilon type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of PKCepsilon by scintillation proximity assayMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(GUINEA PIG)
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.830nMAssay Description:Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response...More data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C epsilon type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of PKCepsilon by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C eta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of PKCeta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C eta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PKCeta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PKCalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C delta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of PKCdelta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C eta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of PKCeta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of PKCbeta-1 by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C epsilon type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of PKCepsilon by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of PKCtheta by scintillation proximity assayMore data for this Ligand-Target Pair

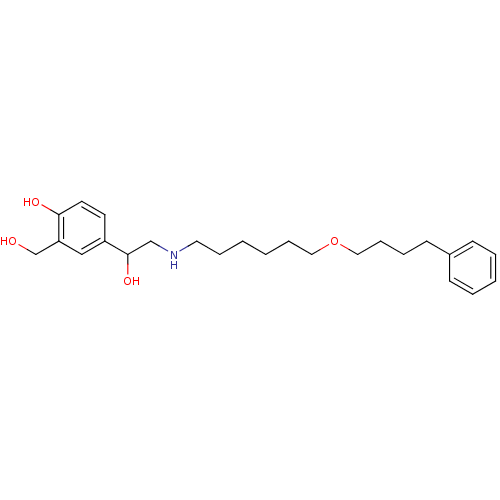
 3D Structure (crystal)
3D Structure (crystal)