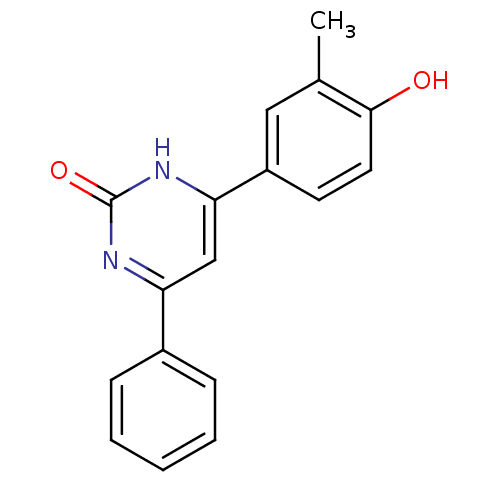
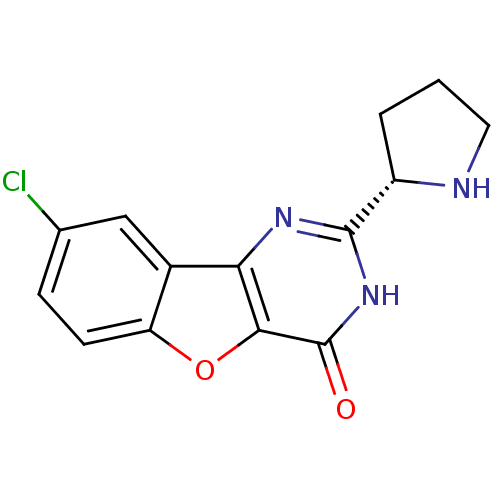
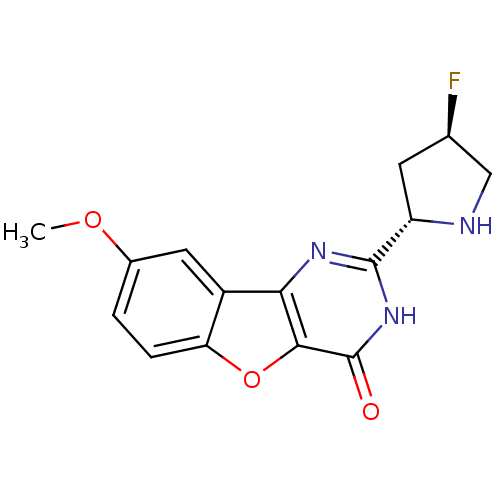
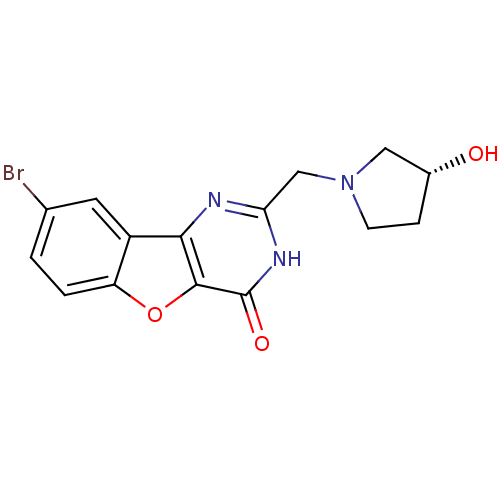
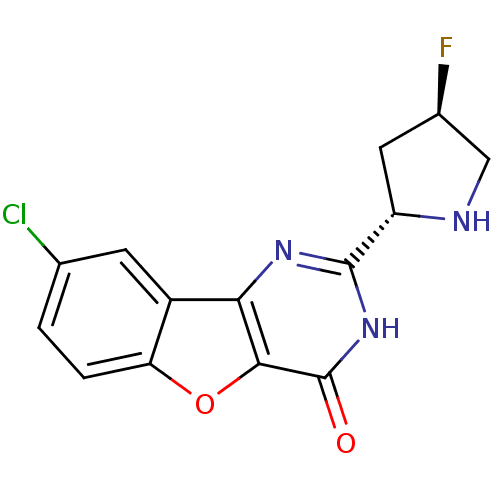
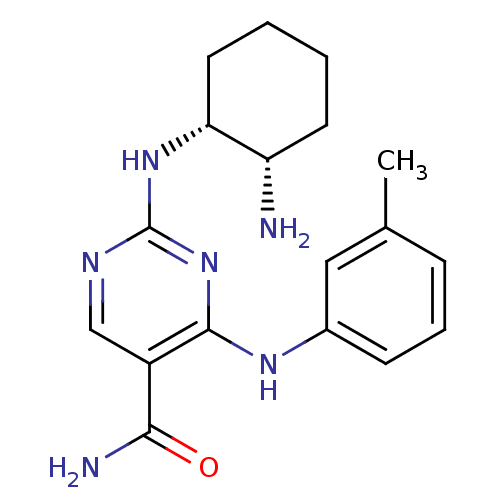
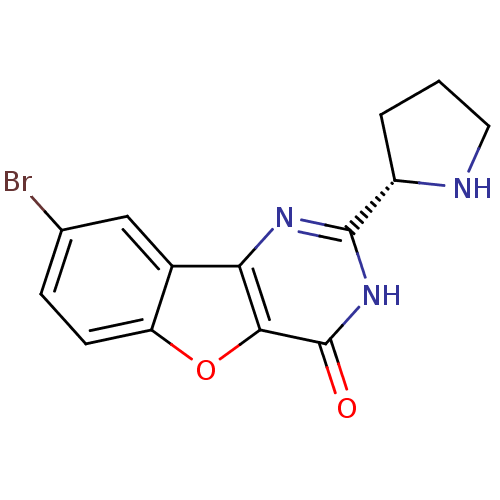
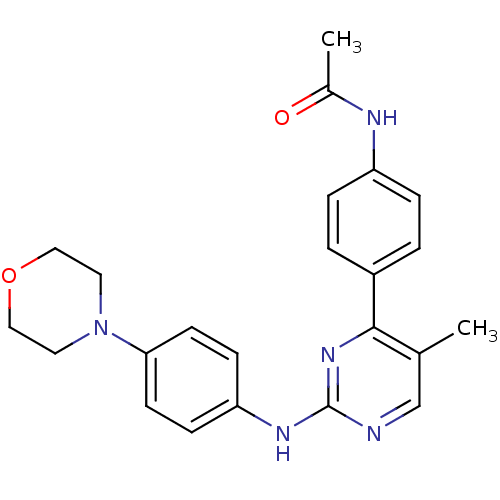
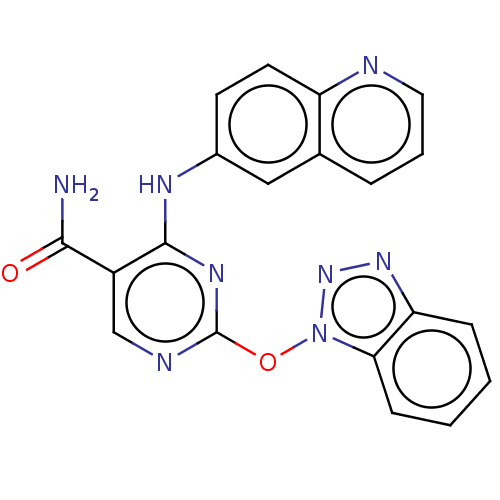
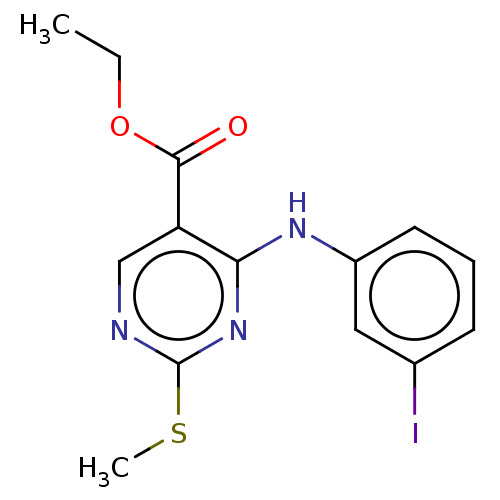
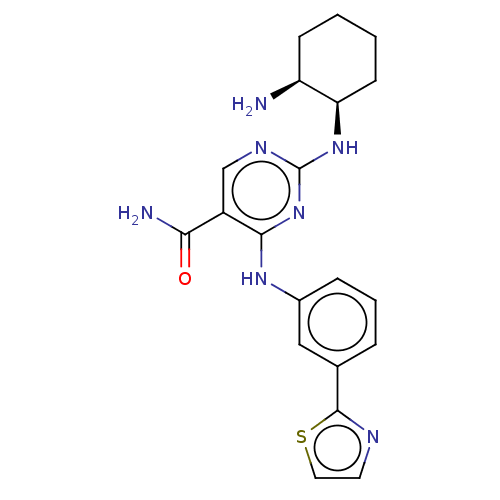
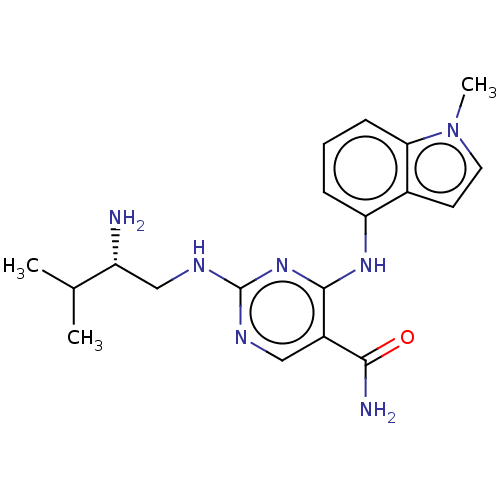
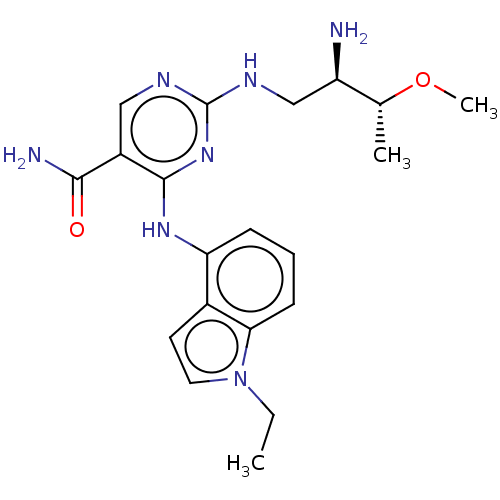
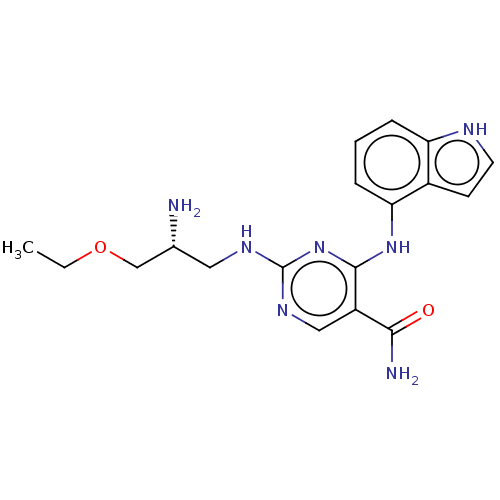
Affinity DataKi: 17.5nMAssay Description:Displacement of [3H]diprenorphine from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 245nMAssay Description:Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
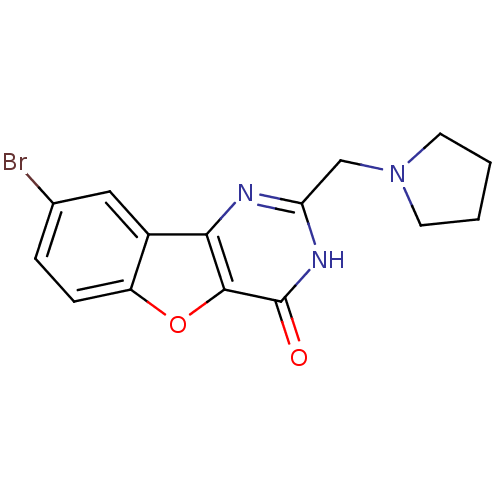
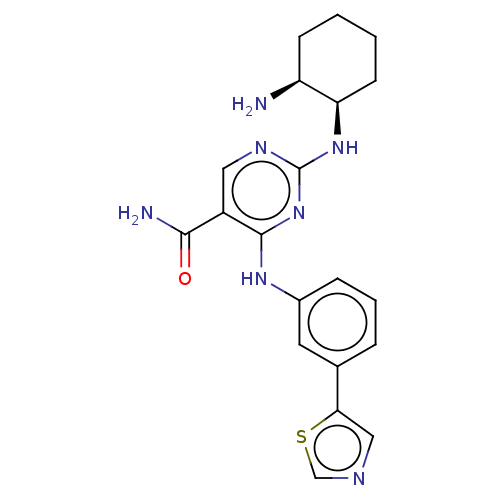
Affinity DataKi: 1.38E+3nMAssay Description:Displacement of [3H]diprenorphine from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >2.50E+4nMAssay Description:Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >2.50E+4nMAssay Description:Displacement of [3H]diprenorphine from mouse delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
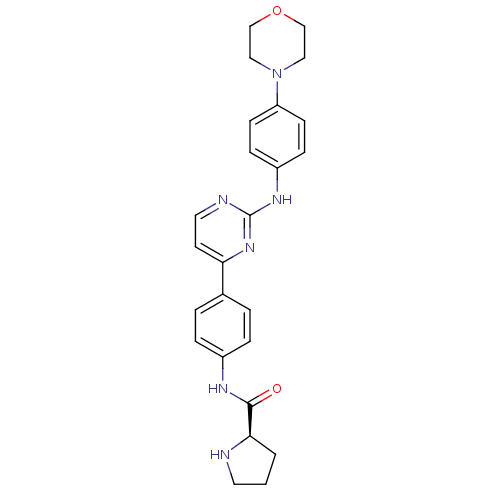
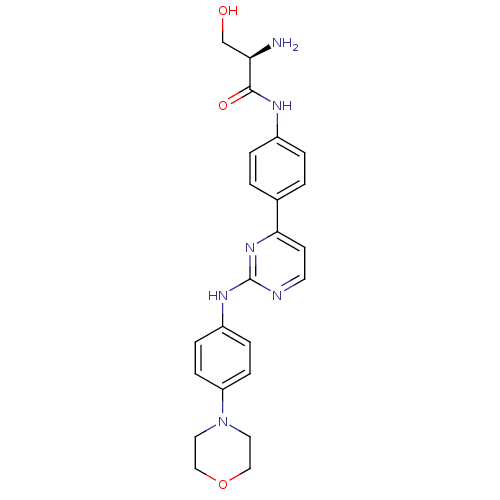
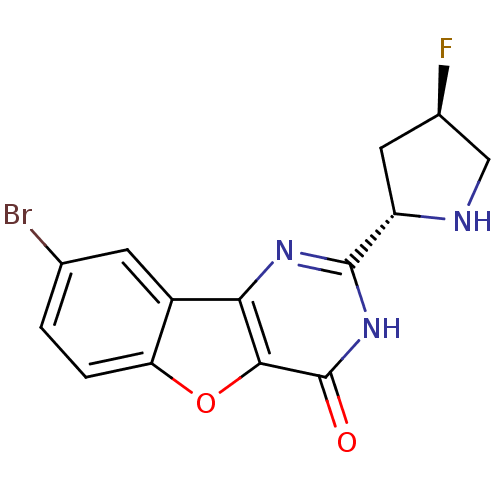
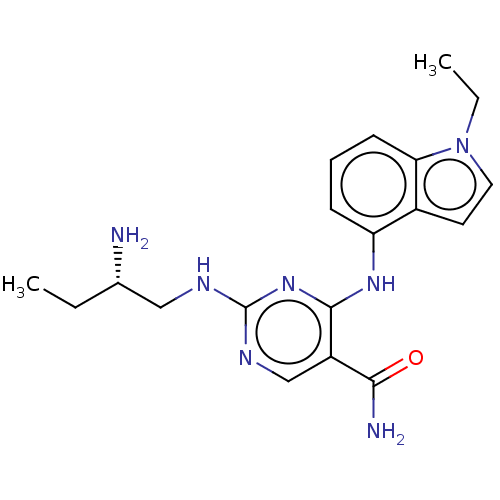
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Employing the Milipore panel of purified kinases EXAMPLE 87 (IC50=1 nM) inhibited 98% of purified Syk kinase activity at 50 nM. IC50 values were dete...More data for this Ligand-Target Pair
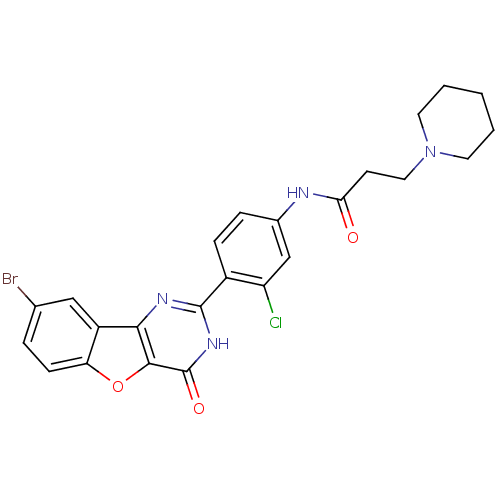
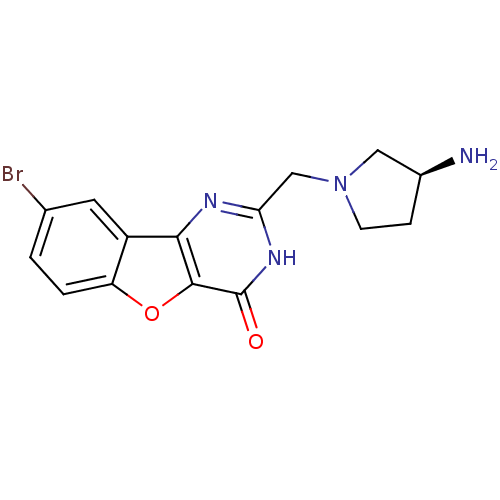
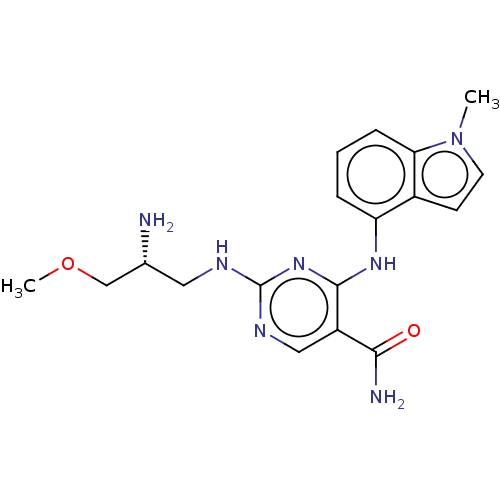
Affinity DataIC50: 1.40nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of N-terminus His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:SYK tyrosine phosphorylation activity is measured using the LANCE Technology developed by Perkin Elmer Life and Analytical Sciences (Boston, Mass.). ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMpH: 7.5 T: 2°CAssay Description:Potency of candidate molecules for inhibiting syk tyrosine phosphorylation activity is assessed by measuring the ability of a test compound to inhibi...More data for this Ligand-Target Pair

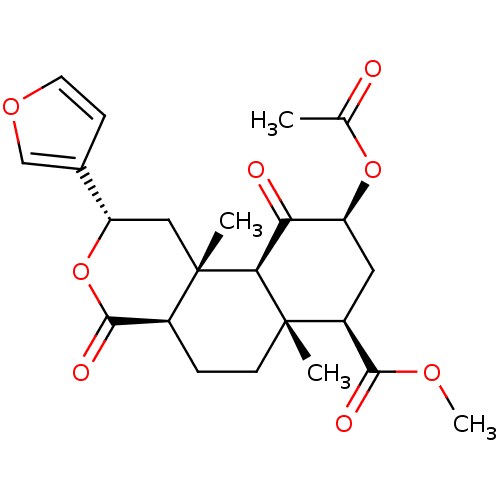
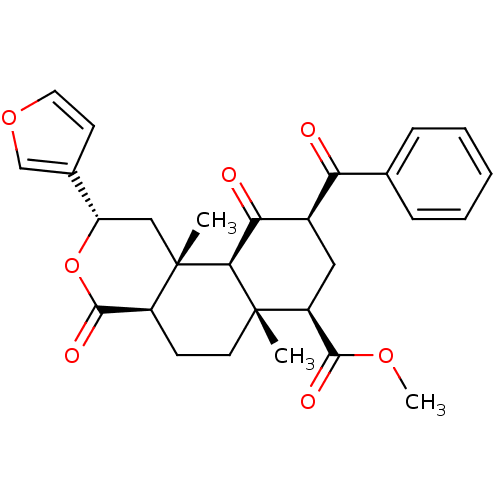
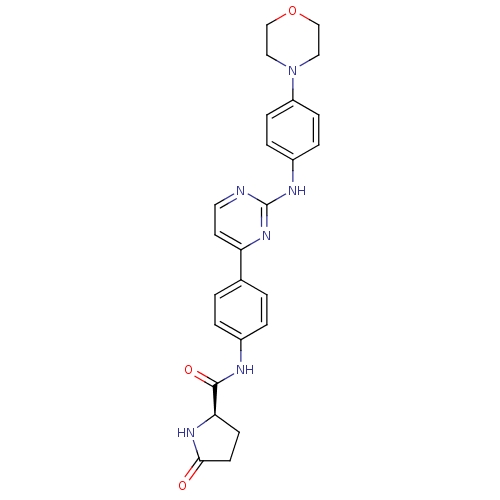
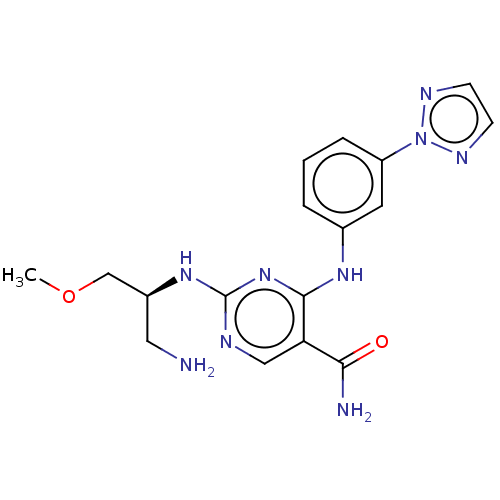

 3D Structure (crystal)
3D Structure (crystal)