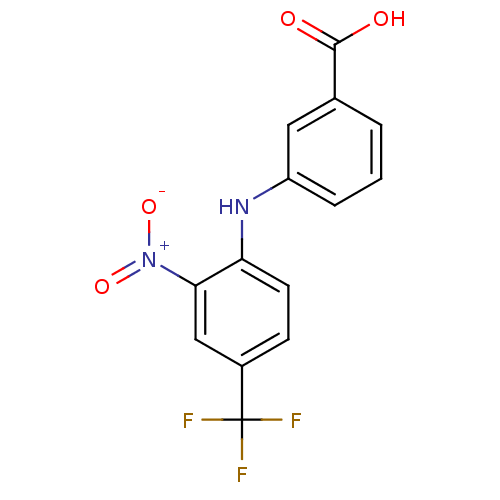
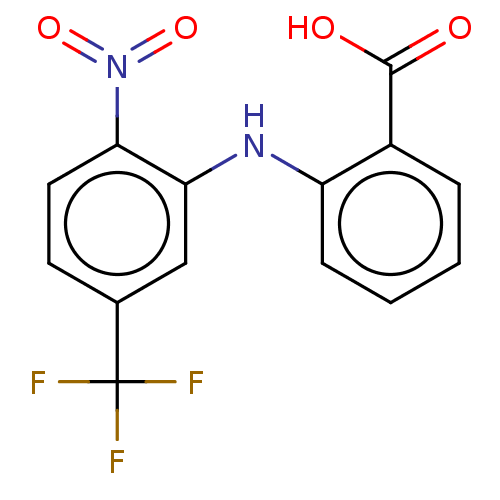
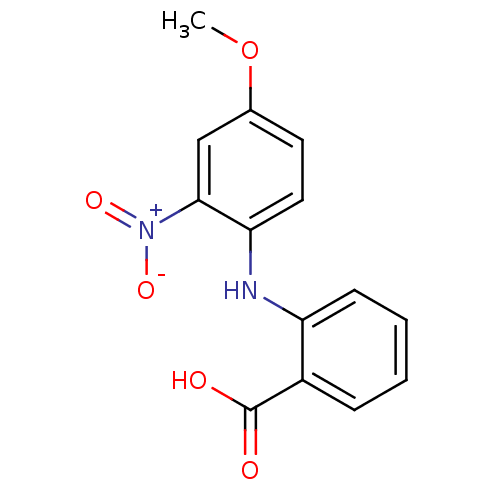
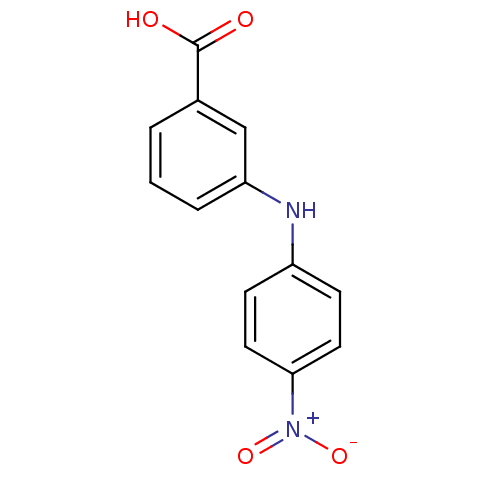
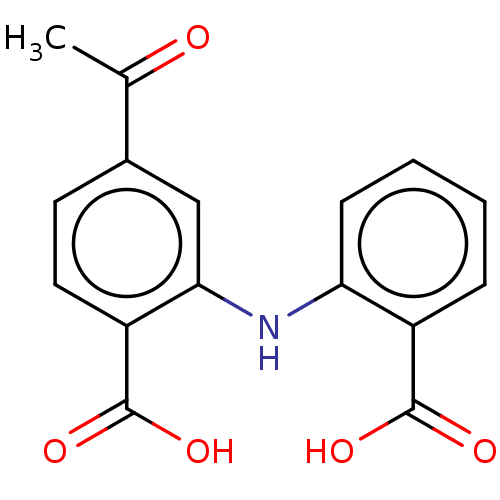
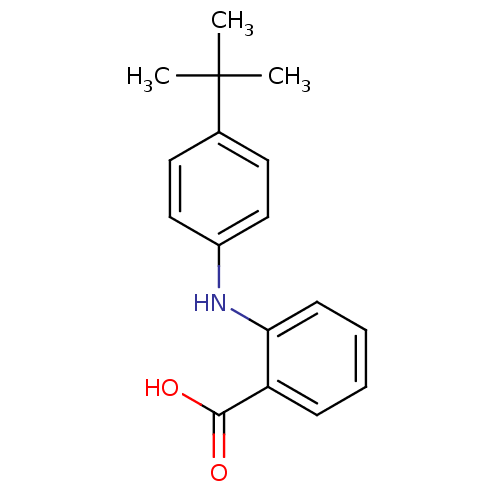
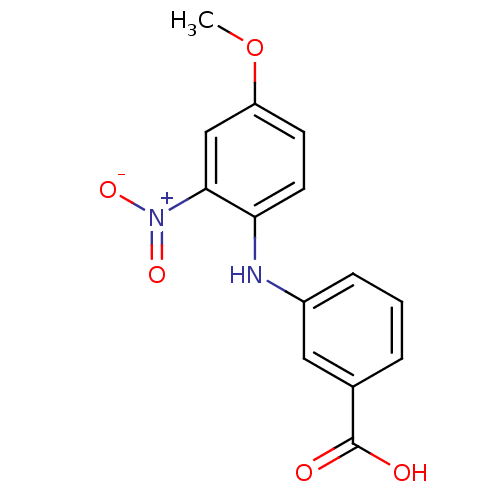
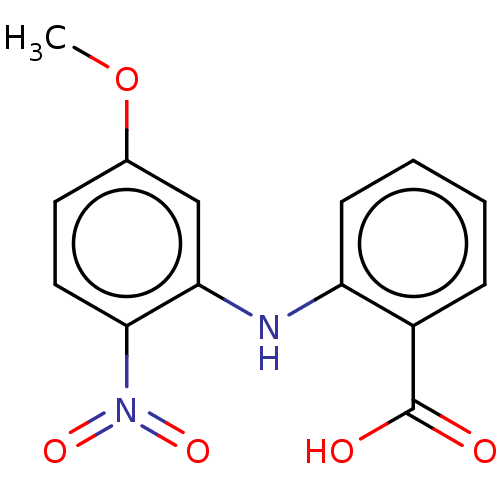
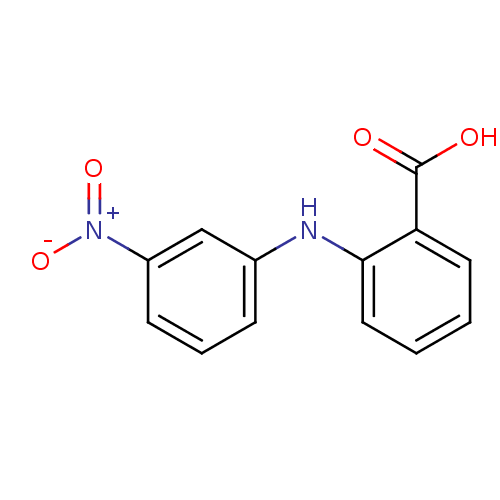
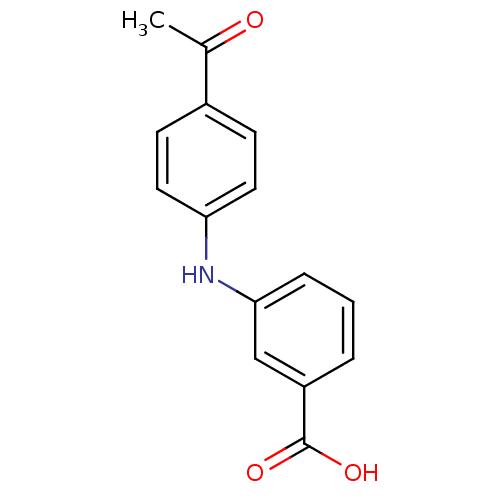
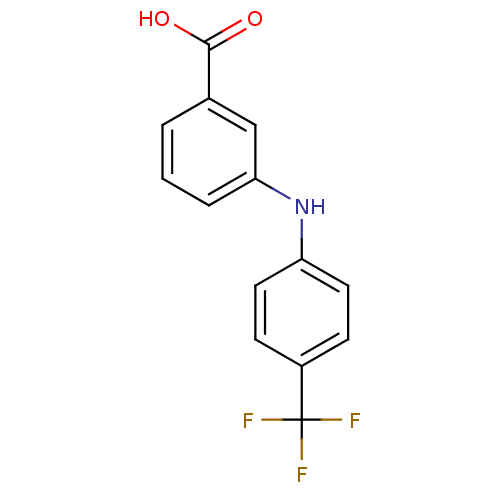
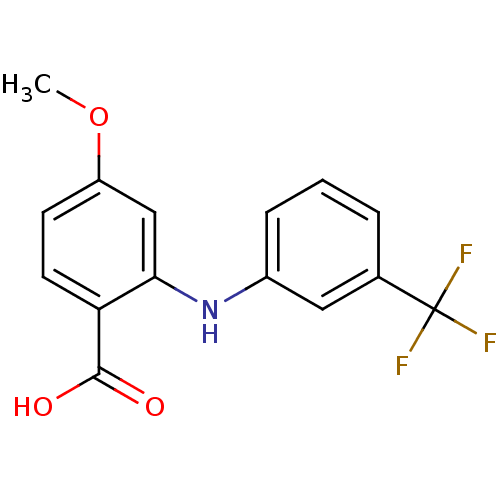
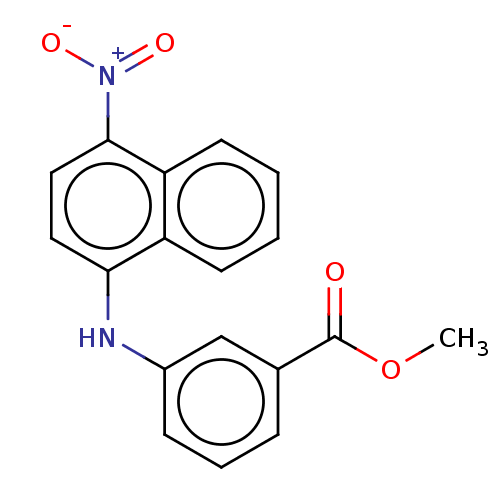
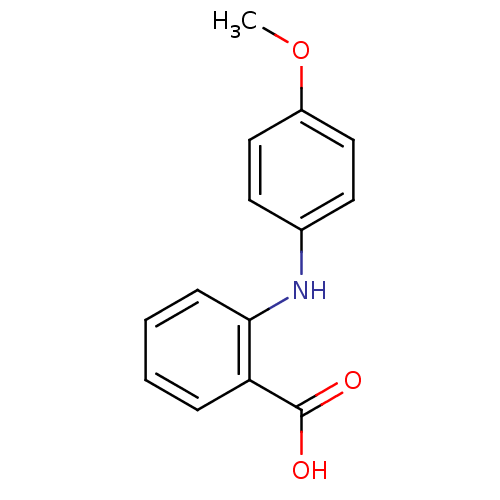
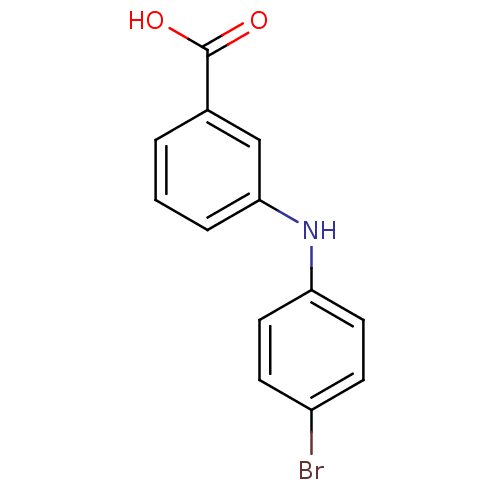
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
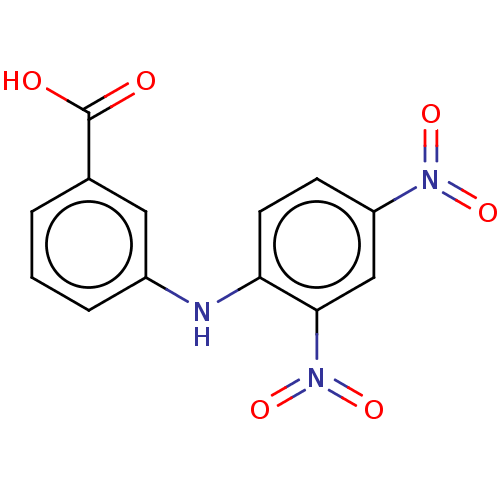
Affinity DataKi: 6.90nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 380nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.32E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.66E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.00E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 8.20E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.10E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.06E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 16nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 16nMAssay Description:Inhibition of COX2 expressed in baculovirus infected SF-21 cells assessed as formation of PGH2 from PGG2 using arachidonic acid as substrate preincub...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 20nMAssay Description:Inhibition of recombinant COX2More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant AKR1B1 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 30nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 36nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 36nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 51nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 51nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 54nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 54nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 62nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 62nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 70nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant AKR1C3 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 80nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 90nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataIC50: 120nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)