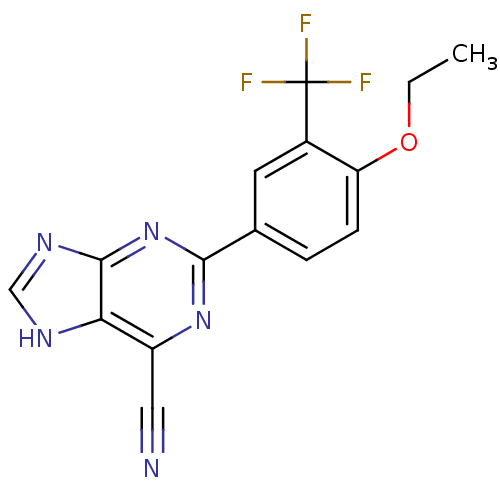
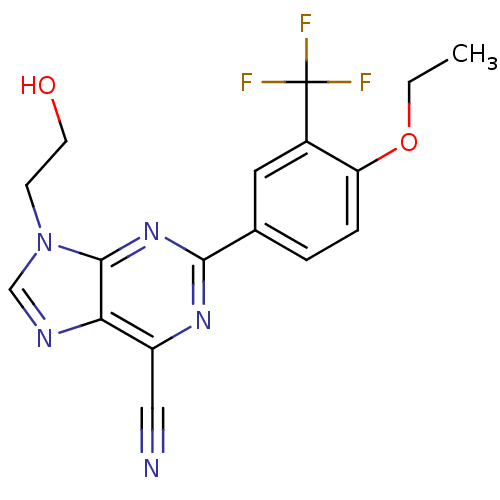
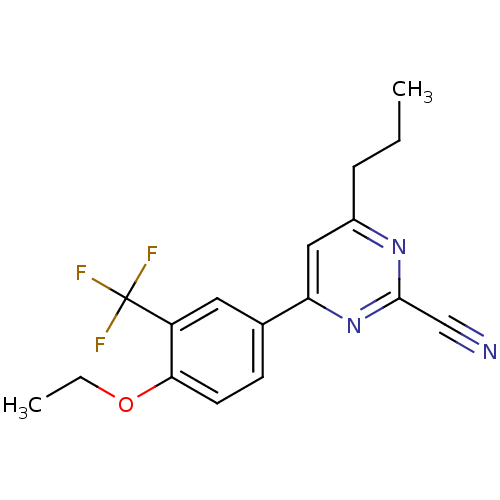
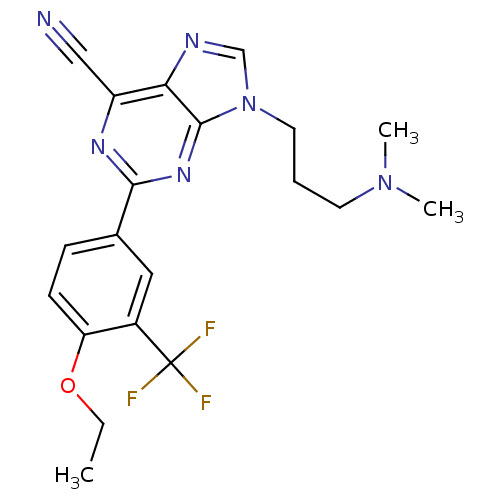
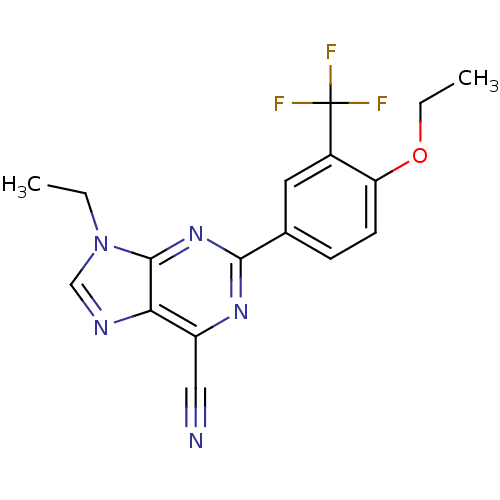
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
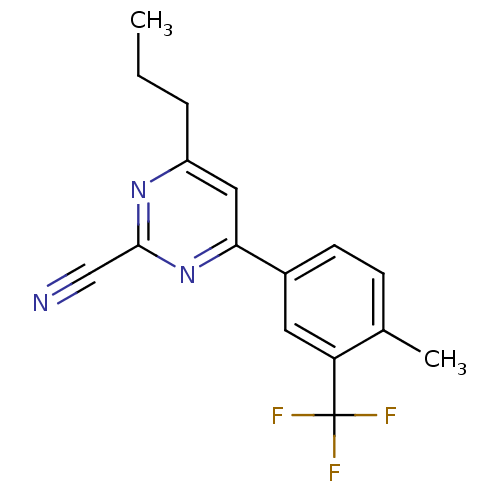
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 370nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 500nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 560nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 630nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 710nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.12E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.58E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.51E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of human ERG channel expressed in HEK293 cells by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.16E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.16E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.98E+3nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.10E+4nMAssay Description:Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant cathepsin K by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human recombinant cathepsin SMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant cathepsin K by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant cathepsin K by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human recombinant cathepsin SMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human recombinant cathepsin SMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of human recombinant cathepsin SMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20nMAssay Description:Inhibition of human recombinant cathepsin SMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant cathepsin K using Z-Phe-Arg-MCA substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant cathepsin SMore data for this Ligand-Target Pair

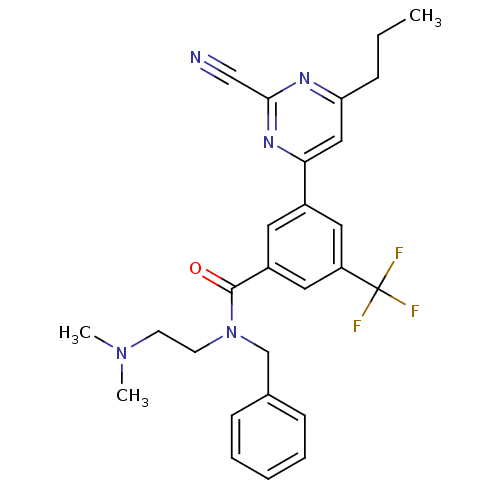


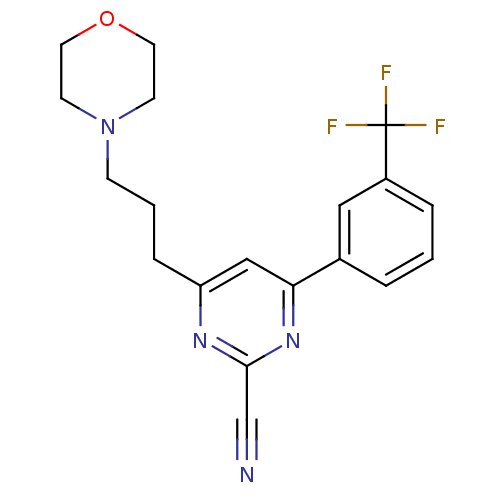
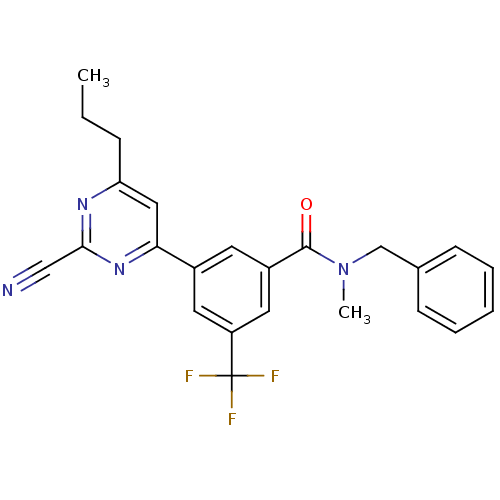
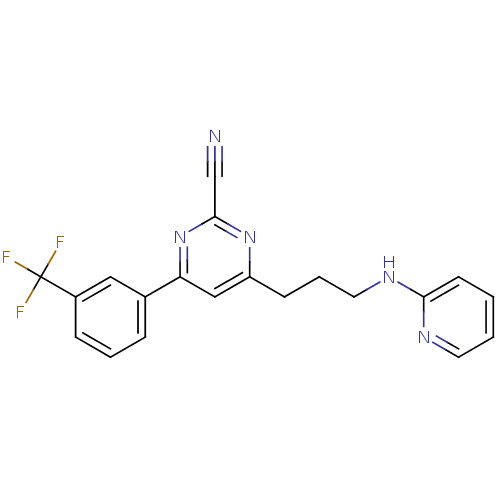

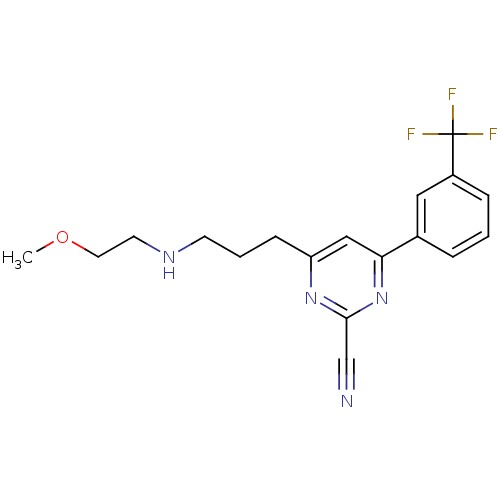
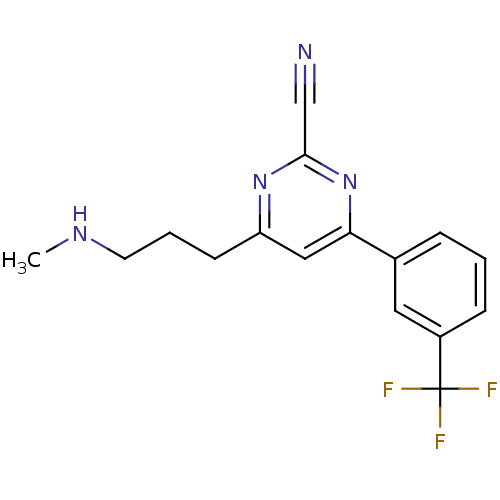

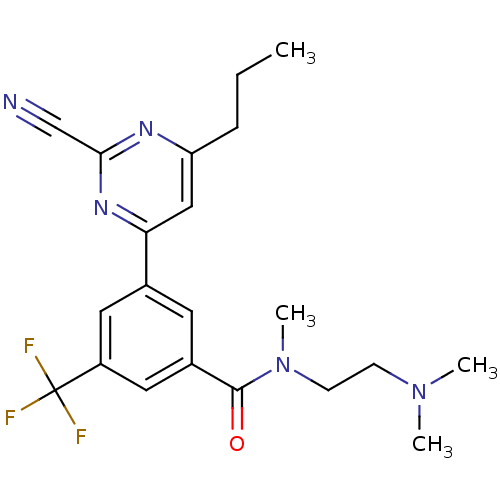
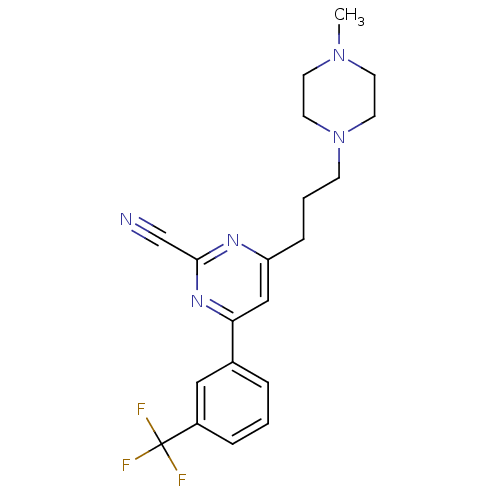
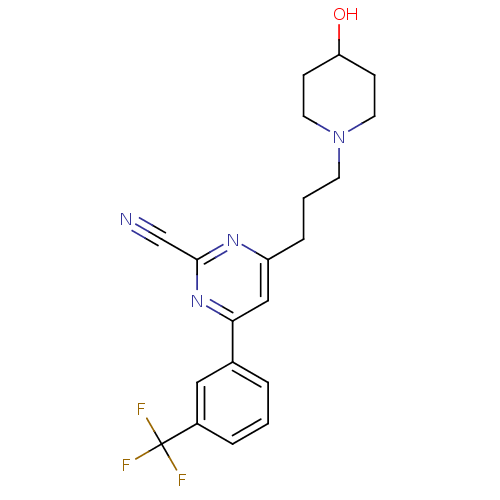

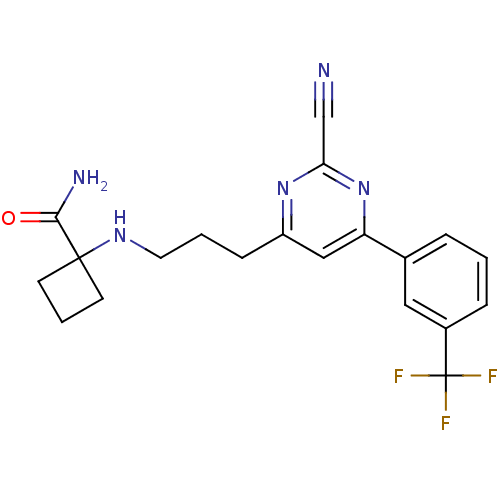
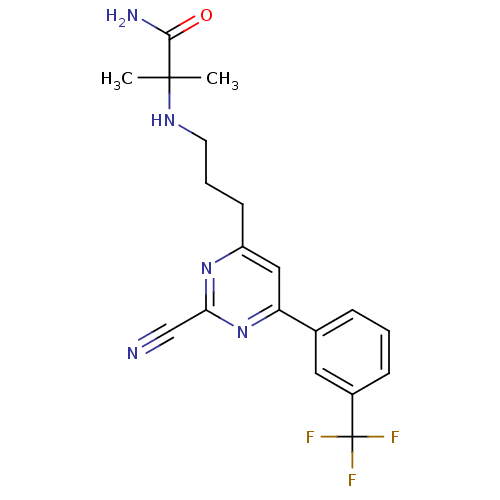

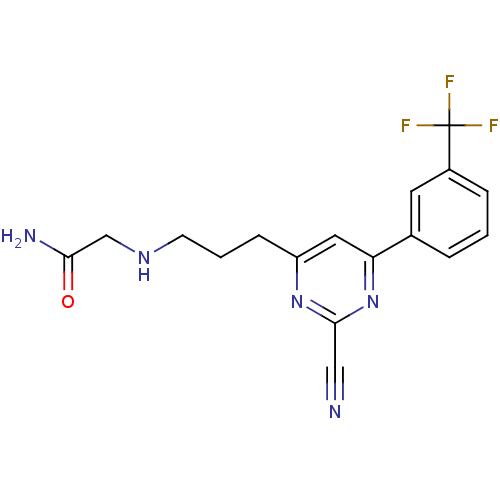
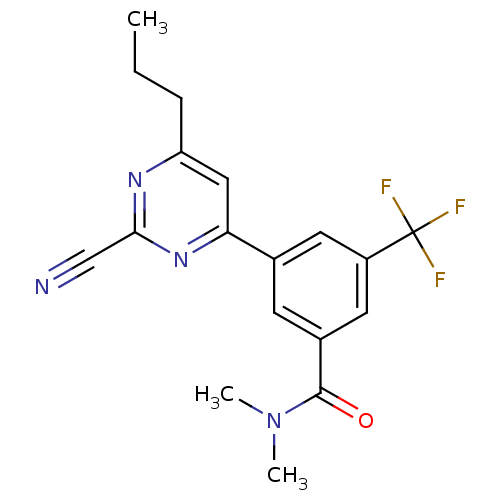
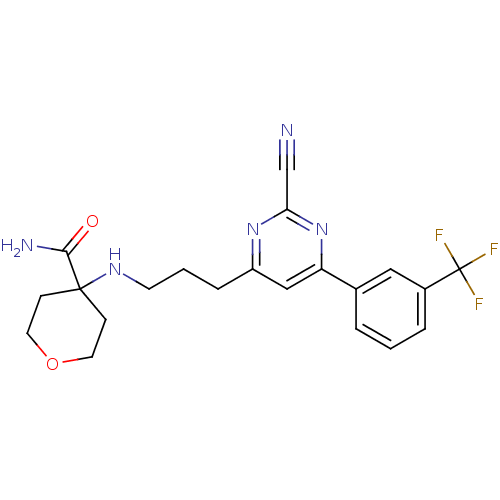
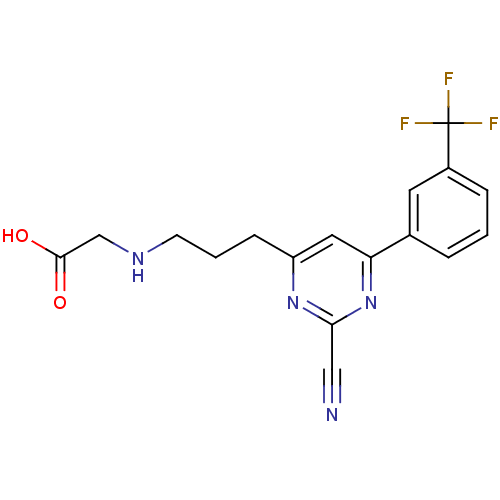
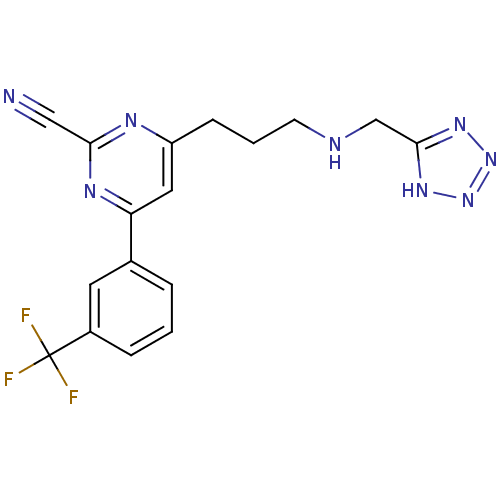


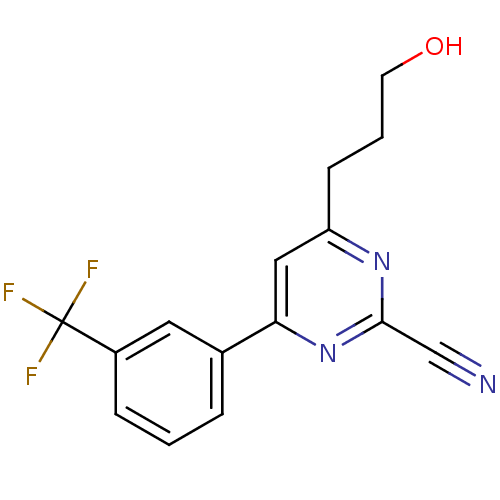

 3D Structure (crystal)
3D Structure (crystal)