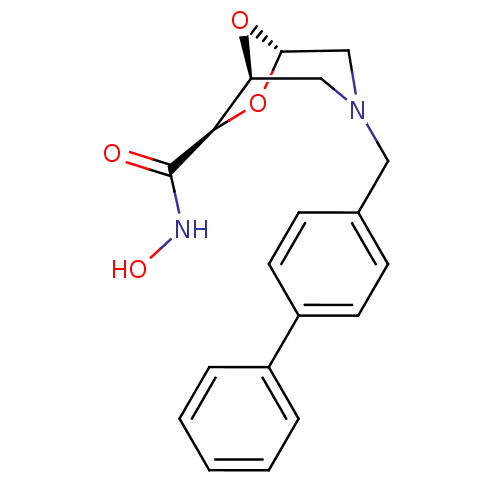
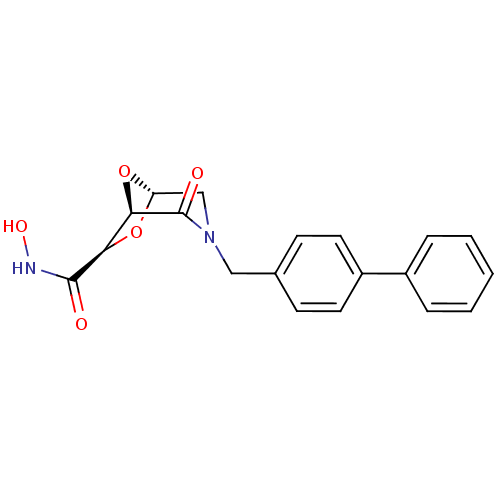
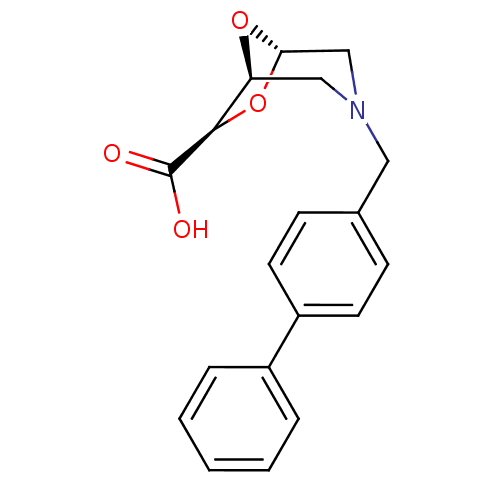
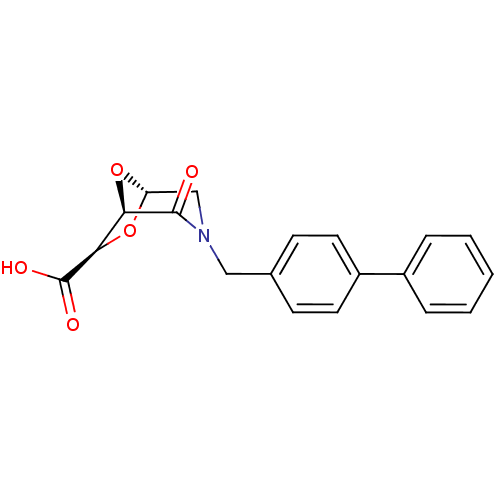
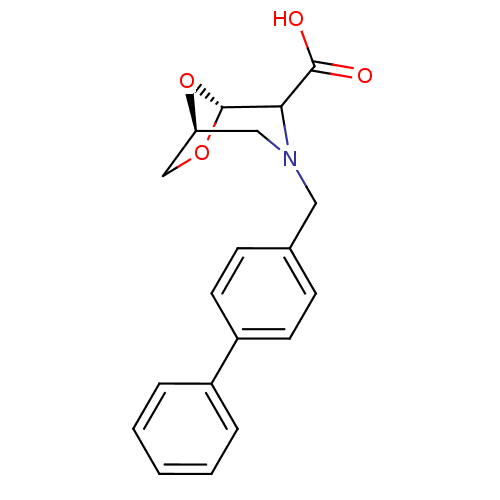
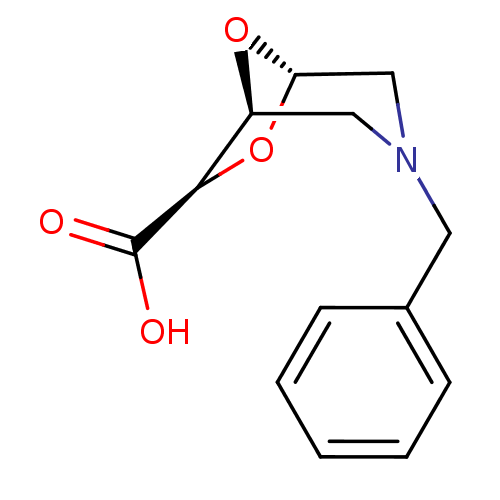
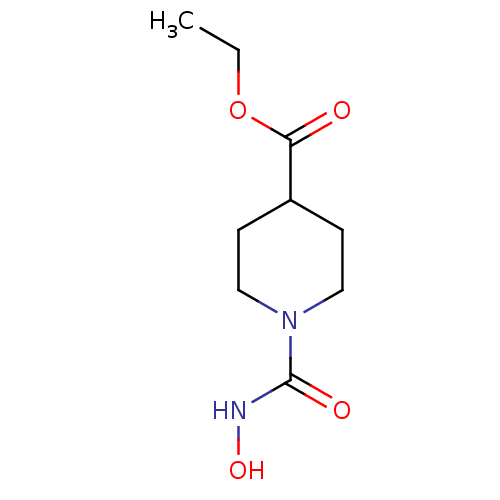
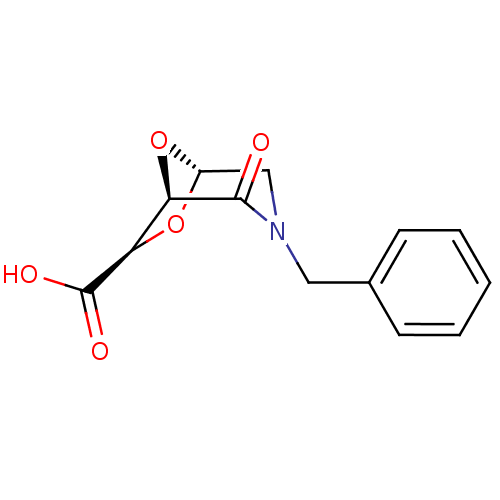
Affinity DataIC50: 1.49E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.49E+5nM Kd: 1.54E+5nMpH: 7.0 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataIC50: 3.99E+5nM Kd: >5.00E+5nMpH: 7.0 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataIC50: 4.25E+5nM Kd: >5.00E+5nMpH: 7.0 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataIC50: 5.13E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.78E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.35E+5nM Kd: >1.00E+6nMpH: 7.0 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataIC50: 8.65E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.54E+5nM Kd: >1.00E+6nMpH: 7.0 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+6nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+6nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+7nMpH: 7.2 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataKd: 3.40E+6nMpH: 7.2 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+7nMpH: 7.2 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataKd: >1.00E+7nMpH: 7.2 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair

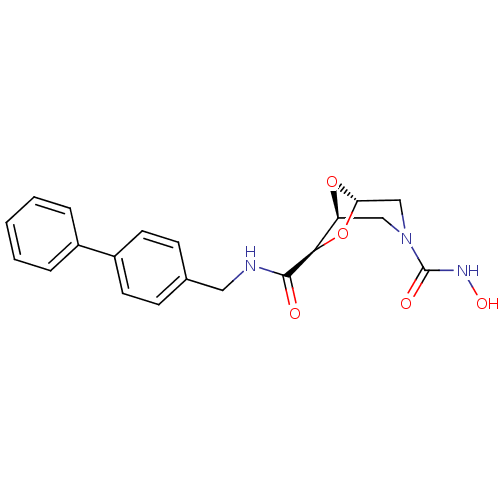
 3D Structure (crystal)
3D Structure (crystal)