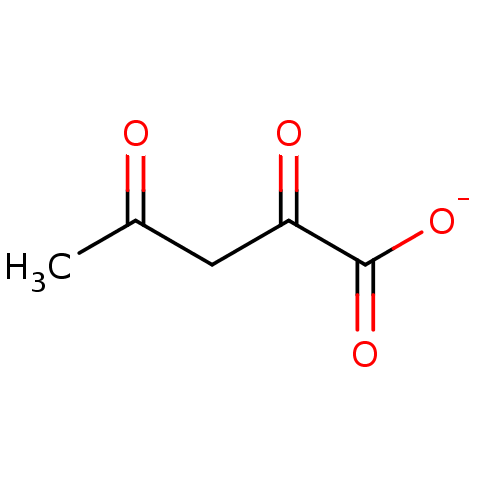
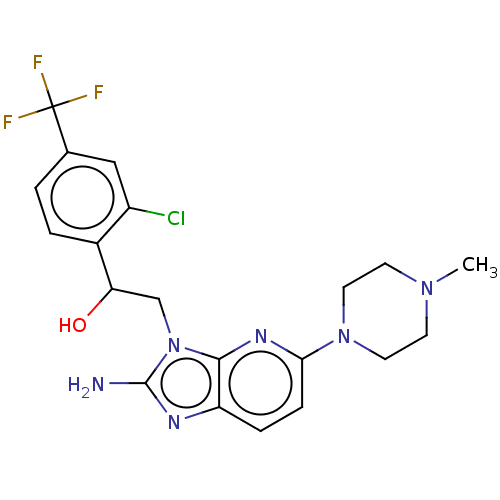
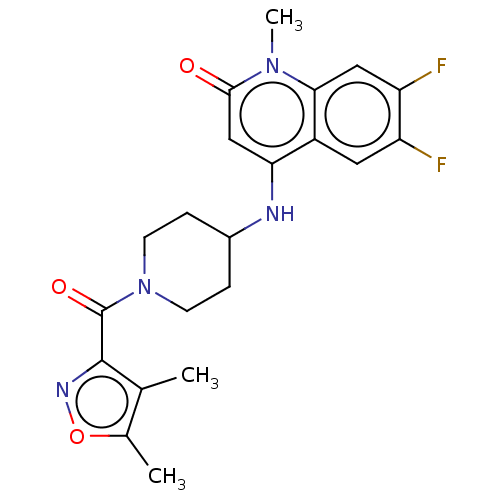
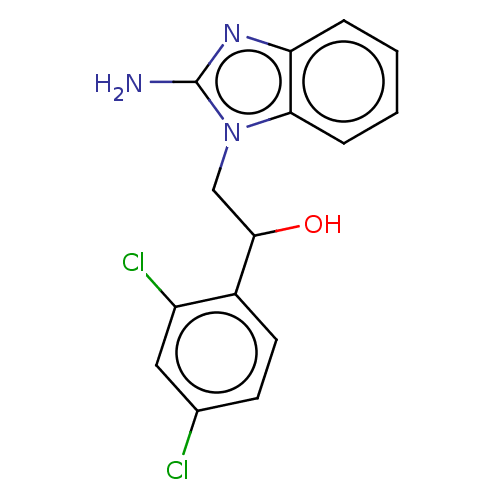
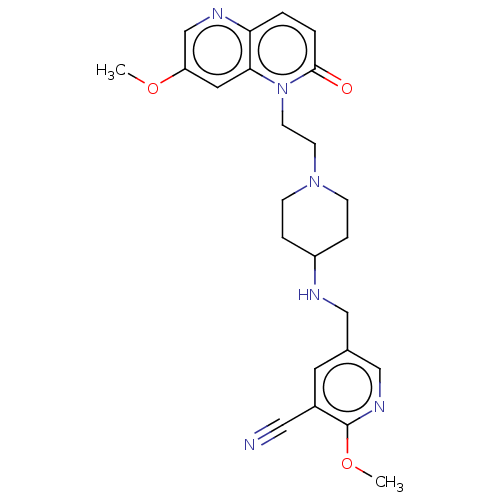
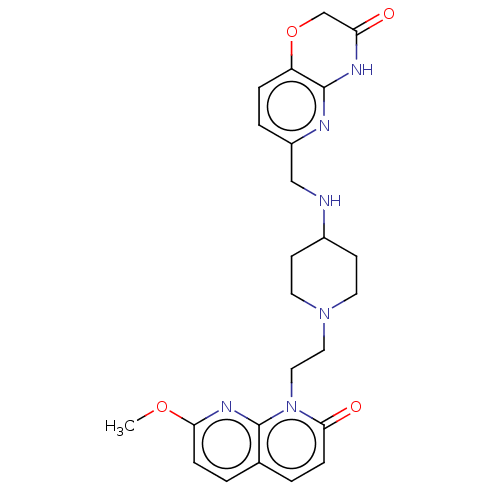
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 4.30E+4nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
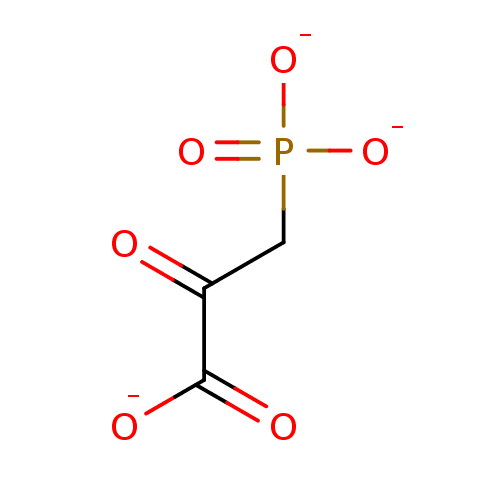
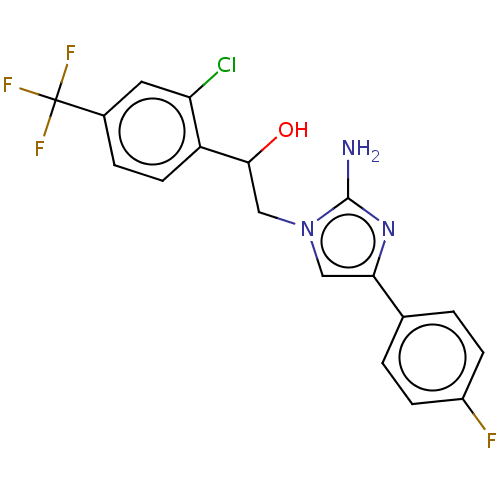
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 4.50E+5nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
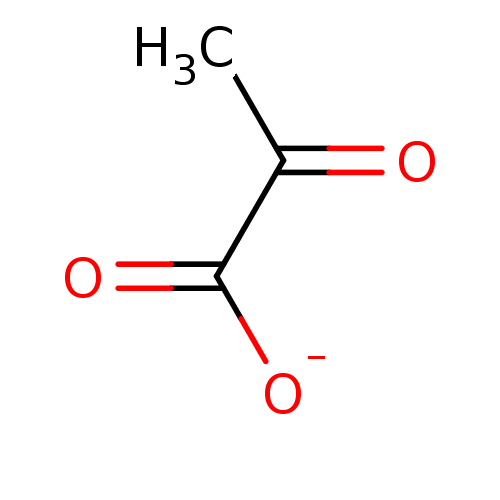
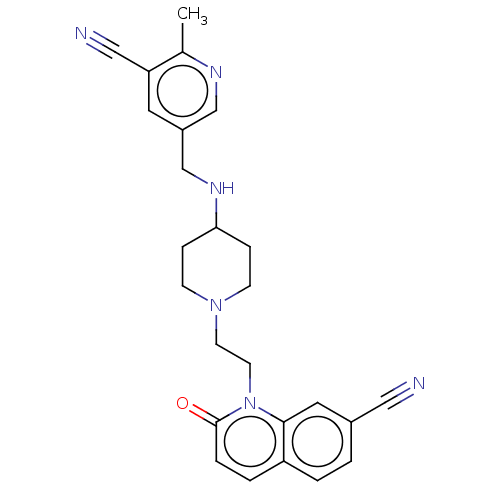
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 5.60E+5nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
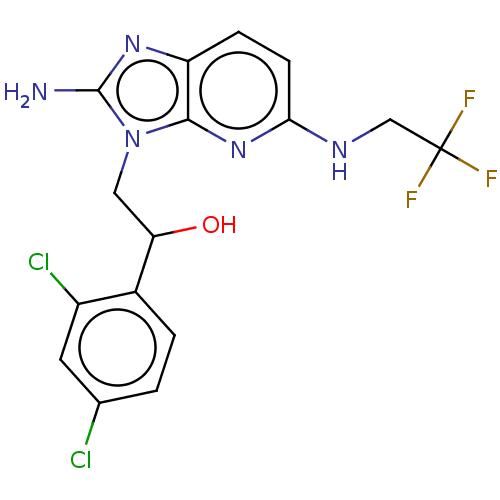
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 1.09E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 3.00E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 3.20E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 6.70E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University of Maryland Biotechnology Institute
University of Maryland Biotechnology Institute
Affinity DataKi: 7.20E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
Affinity DataIC50: 780nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of adenosine A1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of adenosine A1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of adenosine A1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of adenosine A1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.03E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.05E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.22E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of human ERG by medium-throughput electrophysiologyMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 1.66E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.03E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.19E+4nMAssay Description:Inhibition of mu opioid receptor (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of human ERG by patch clamp electrophysiological analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.79E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.79E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.83E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.94E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)