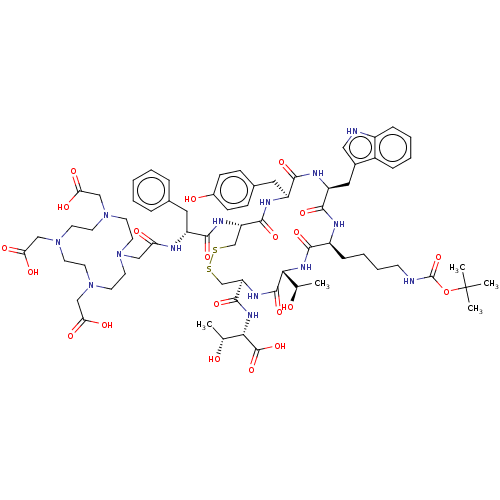
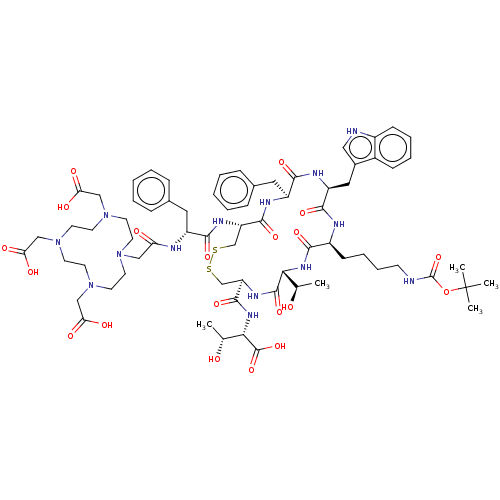
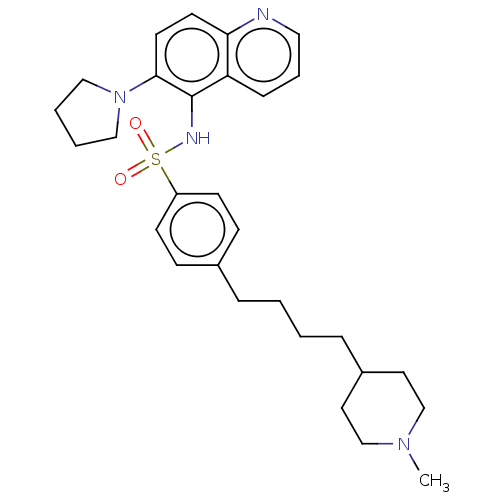
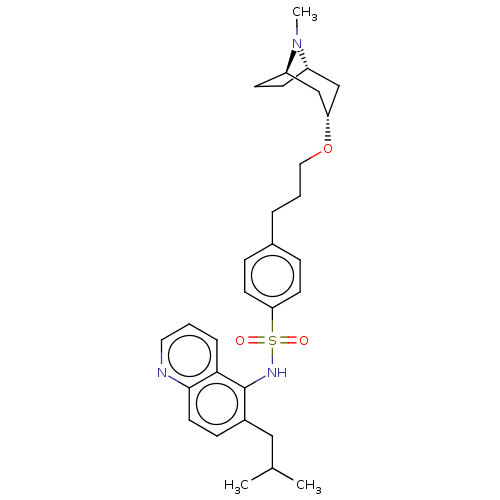
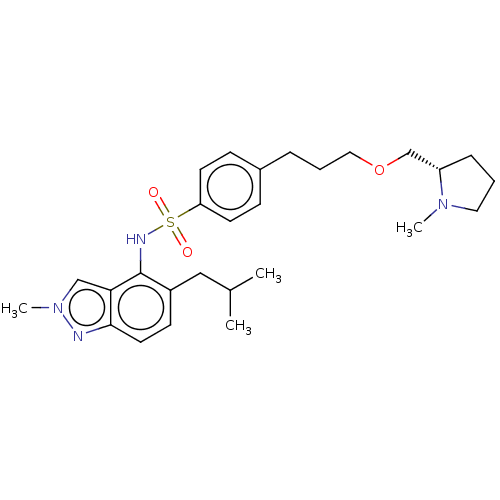
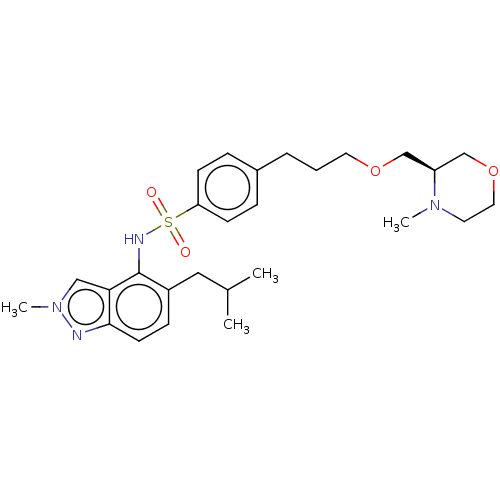
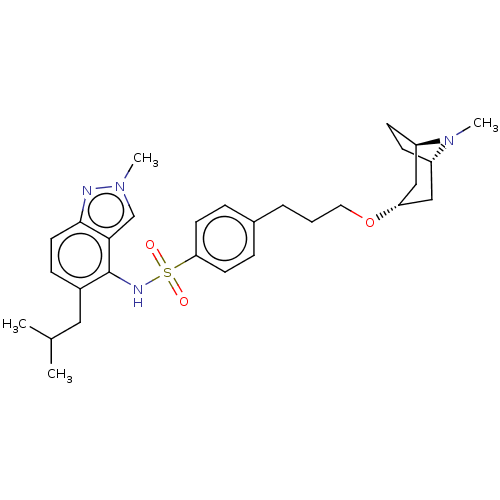
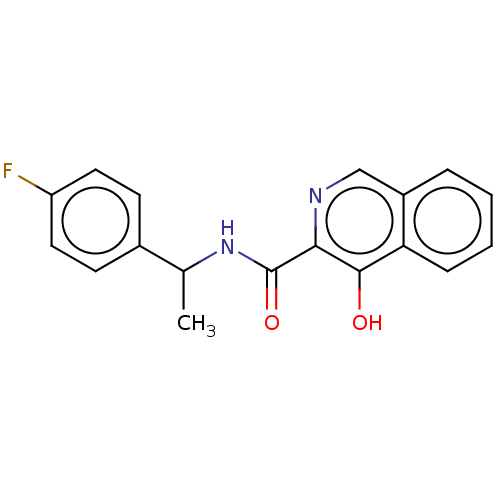
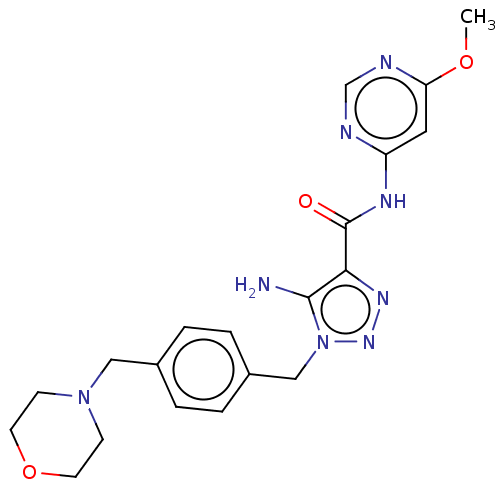
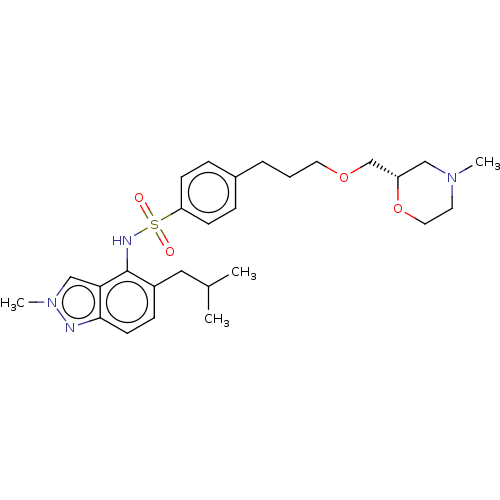
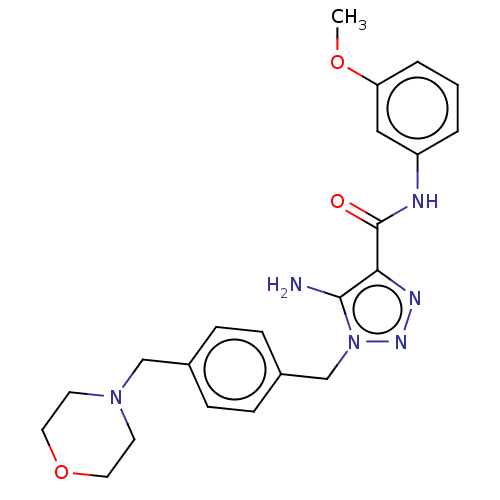
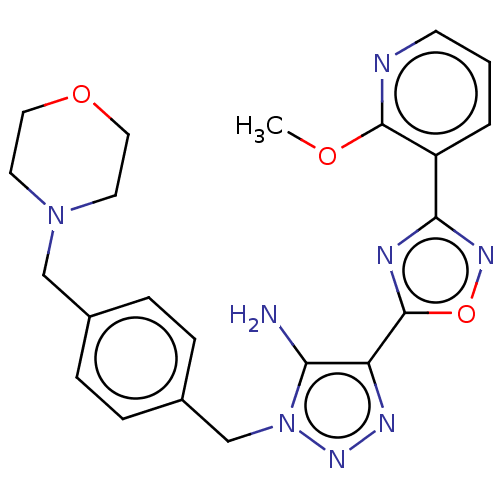
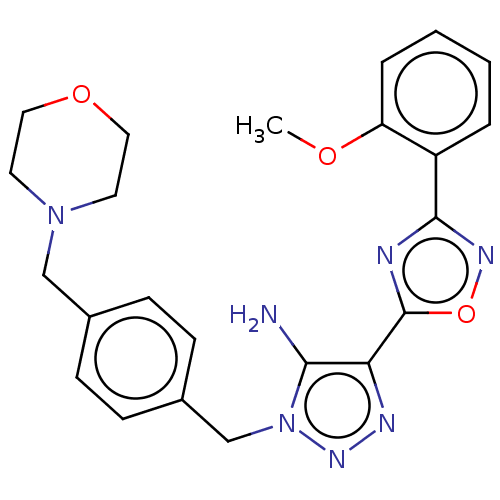
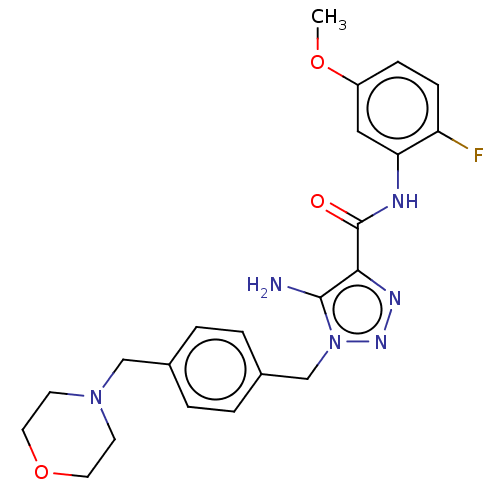
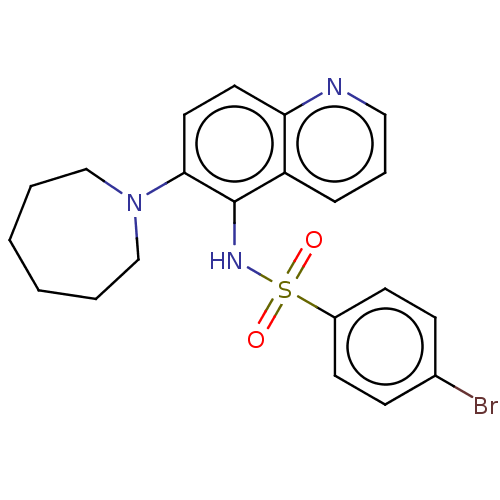
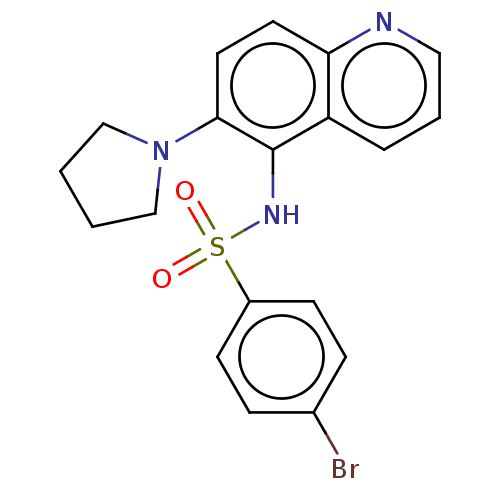
Affinity DataKi: 0.501nMAssay Description:Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligandMore data for this Ligand-Target Pair
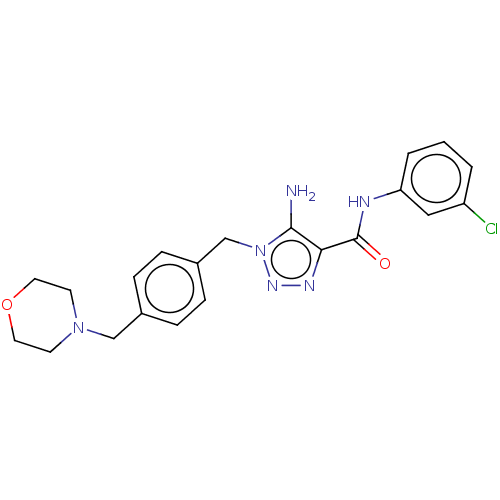
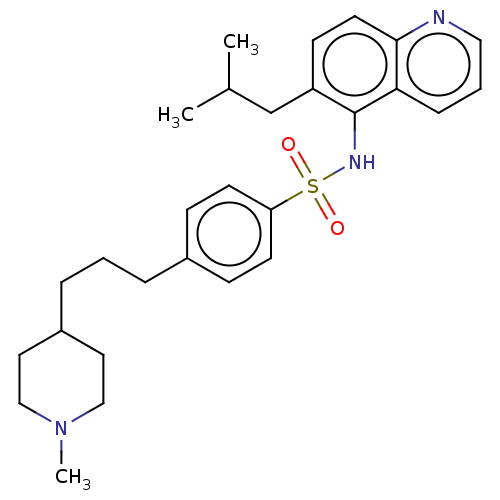
Affinity DataKi: 1.30nMAssay Description:Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligandMore data for this Ligand-Target Pair
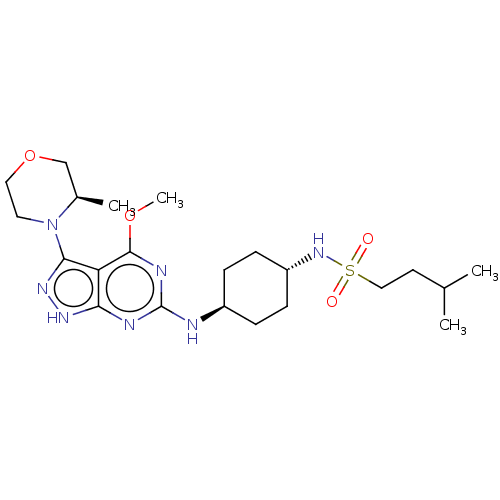
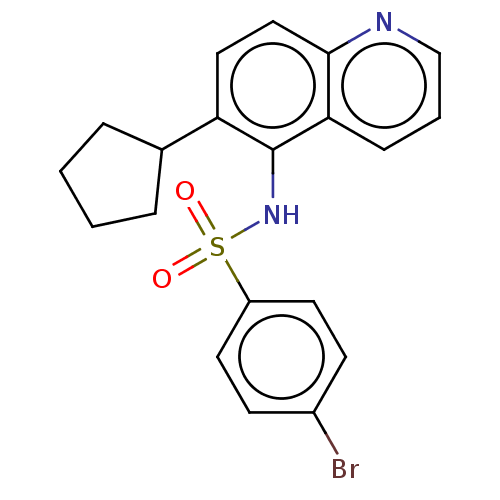
Affinity DataKi: 3.20nMAssay Description:Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligandMore data for this Ligand-Target Pair
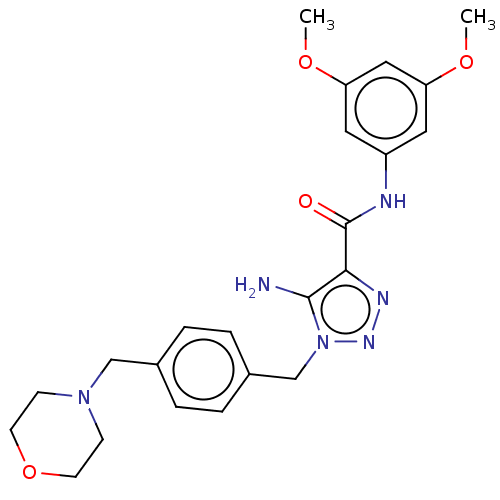
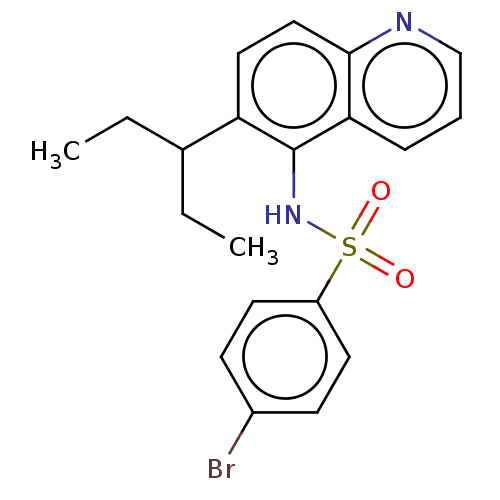
Affinity DataIC50: 1nMAssay Description:Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using Y-labelled SMT487 radioligandMore data for this Ligand-Target Pair
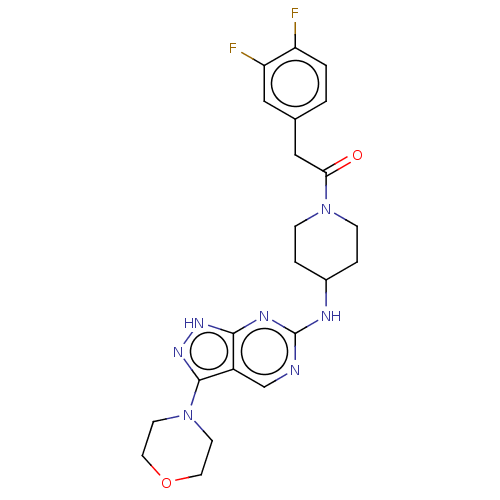
Affinity DataIC50: 110nMAssay Description:Inhibition of human ERK8 (2 to 544 residues) incubated for 5 mins in presence of [gamma33P]ATP by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 114nMAssay Description:Inhibition of CDK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 199nMAssay Description:Inhibition of CDK4 (unknown origin)More data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 550nMAssay Description:Inhibition of human p38beta MAPK (1 to 364 residues) incubated for 5 mins in presence of [gamma33P]ATP by scintillation counting analysisMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
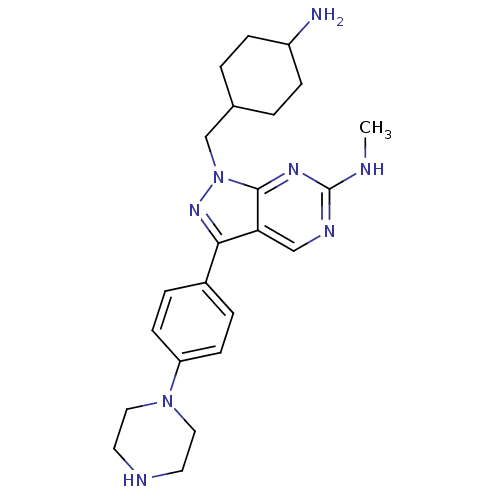
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.98E+3nMAssay Description:Inhibition of MAOA (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
Affinity DataIC50: 6.31E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.75E+4nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human CDK4 by kinobeads-based assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
In DepthDetails
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 3.60E+4nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 4.10E+4nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 4.90E+4nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 6.40E+4nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetGlycylpeptide N-tetradecanoyltransferase 1(Homo sapiens (Human))
University of Dundee
Curated by ChEMBL
University of Dundee
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation proximity assayMore data for this Ligand-Target Pair