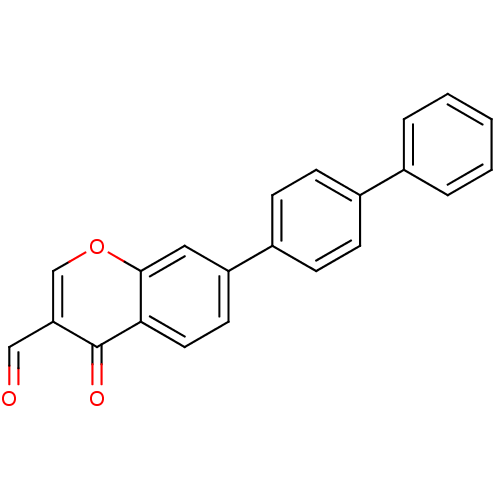
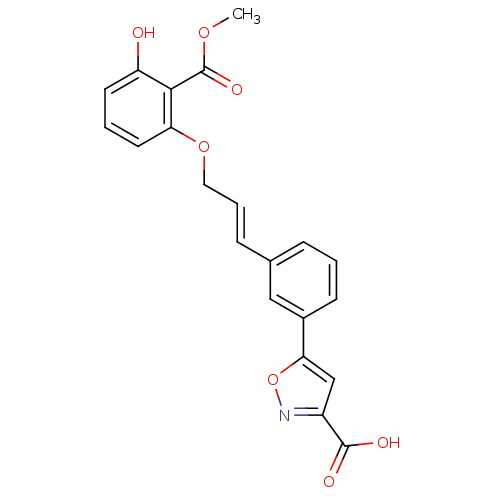
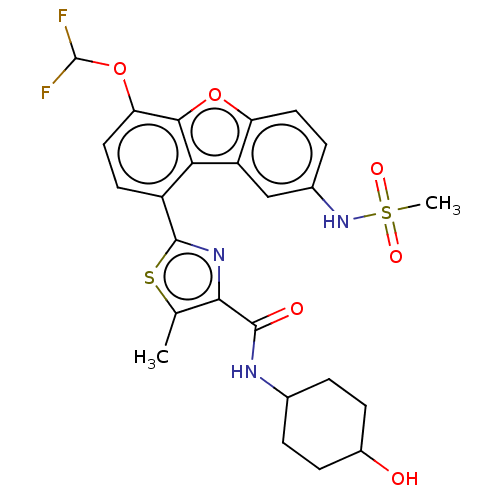
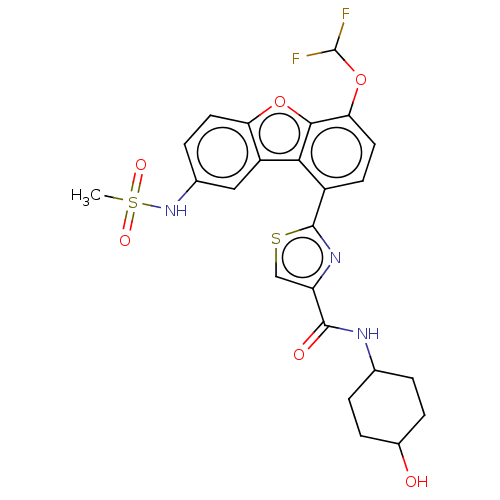
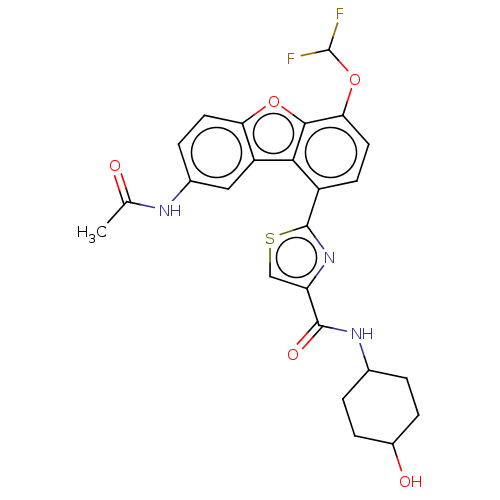
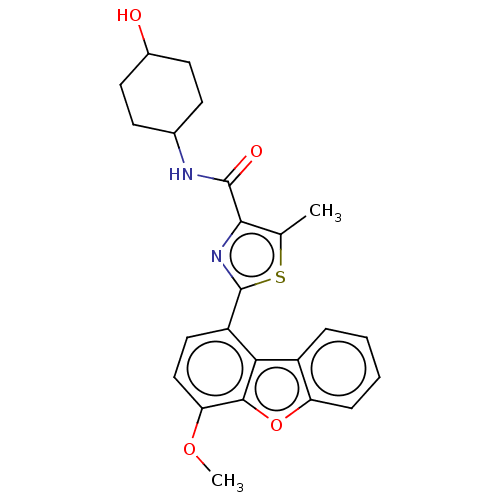
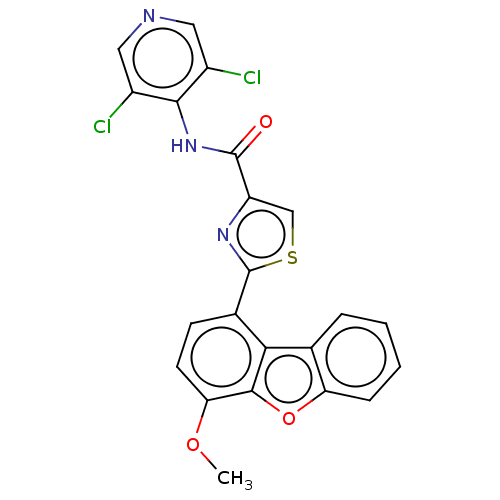
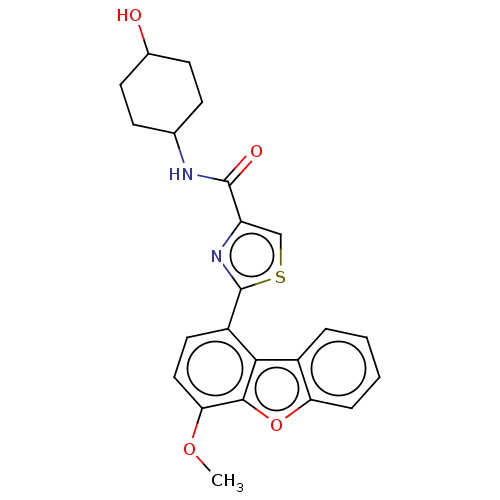
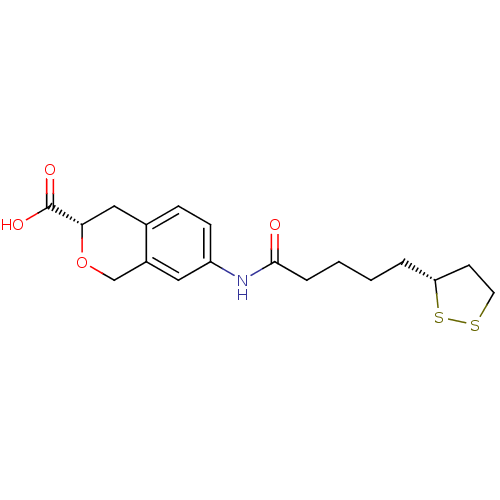
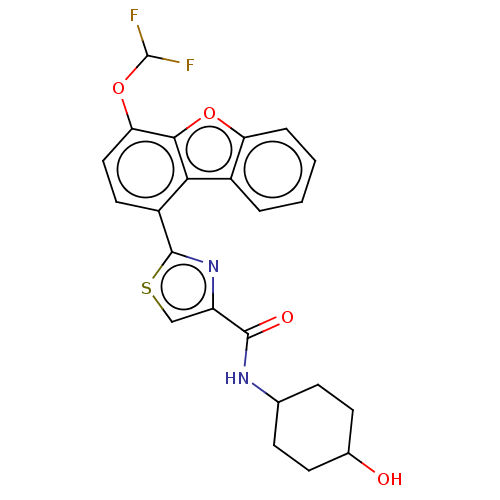
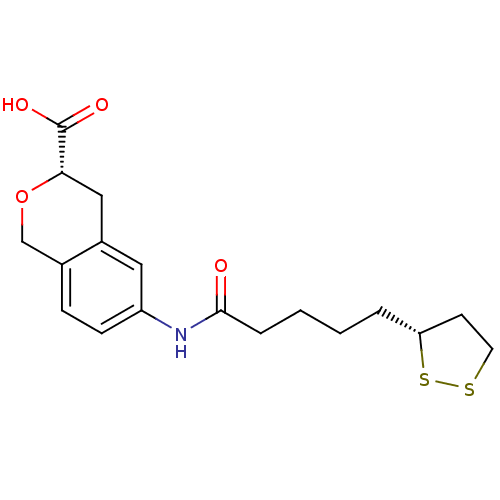
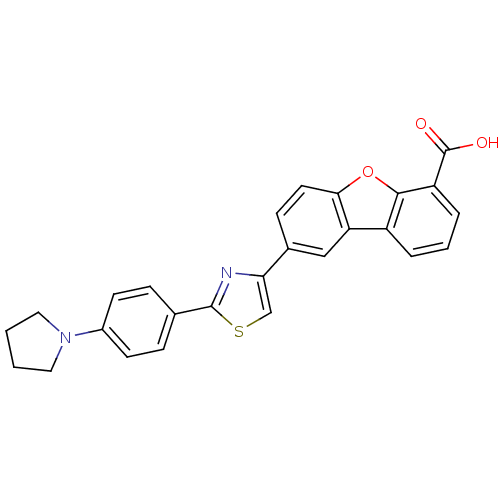
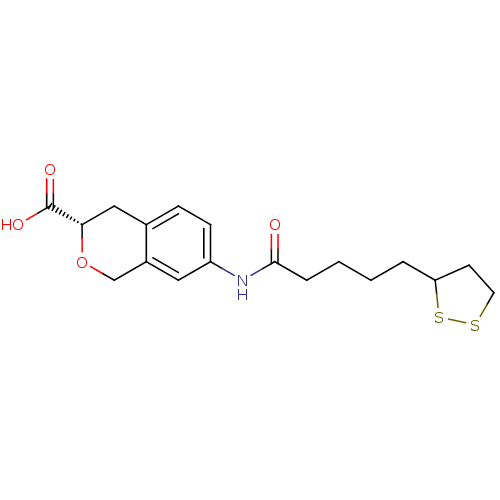
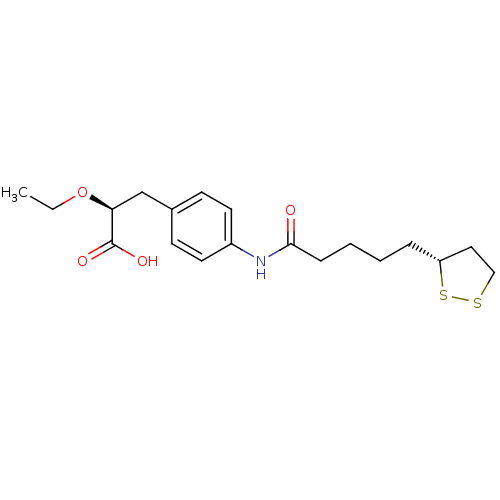
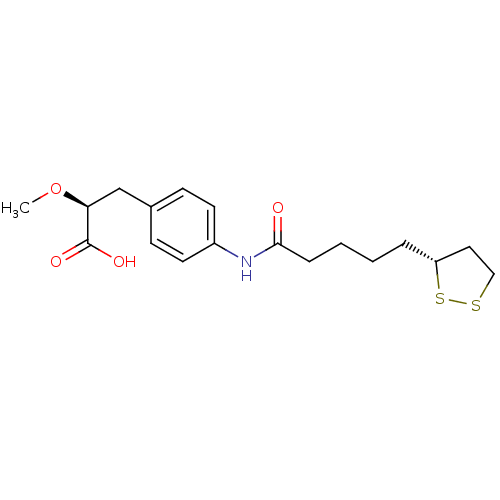
Affinity DataKi: 4.90nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Inhibition of human recombinant TCPTP after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of TCPTPMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
Affinity DataKi: 1.57E+4nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
Affinity DataKi: 2.12E+4nMAssay Description:To evaluate the potency of synthesized compounds against Mpro, the proteolytic activity of 50 nM Mpro -His and Mpro was first measured in the presenc...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 5.10E+4nMAssay Description:Inhibition of human recombinant TCPTP after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 5.10E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 2.02E+5nMAssay Description:Inhibition of TCPTPMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataKi: 2.02E+5nMAssay Description:Inhibition of human recombinant TCPTP after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataIC50: 51.6nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataIC50: 64nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataIC50: 121nMAssay Description:Inhibition of human recombinant PTP1B after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 127nMAssay Description:Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
Affinity DataIC50: 136nMAssay Description:Inhibition of human recombinant TCPTP after 30 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Glenmark Research Center
Curated by ChEMBL
Glenmark Research Center
Curated by ChEMBL

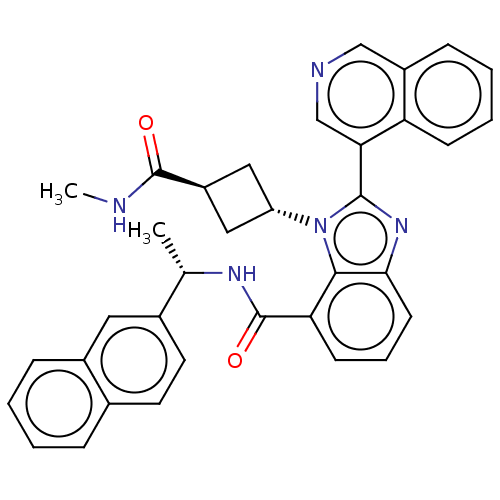

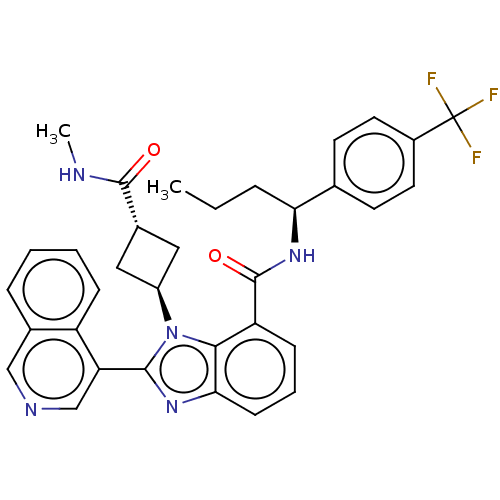
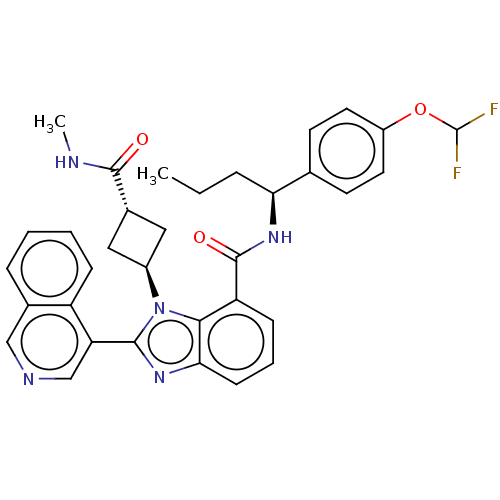

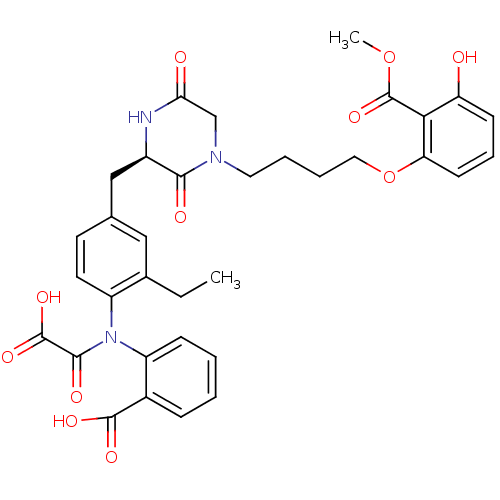
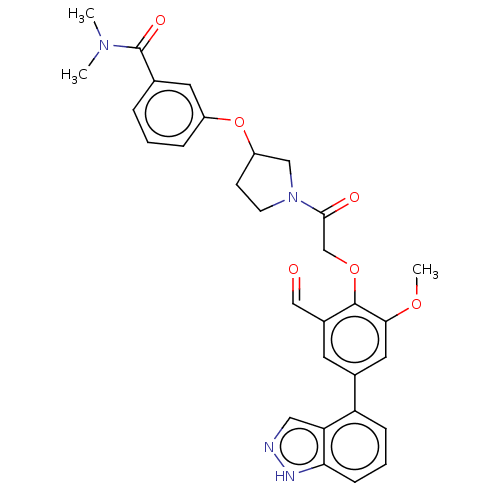
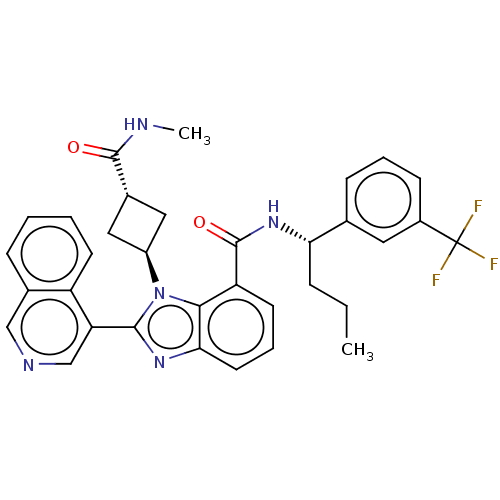
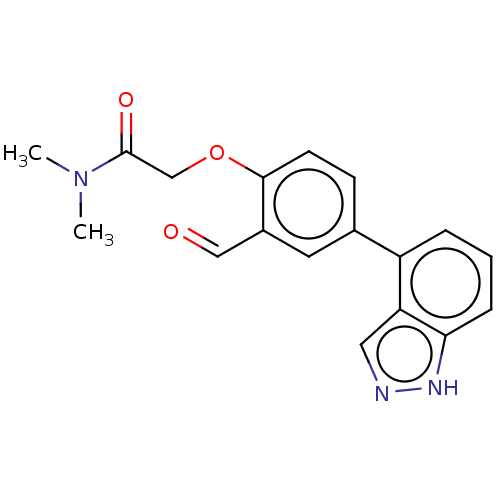
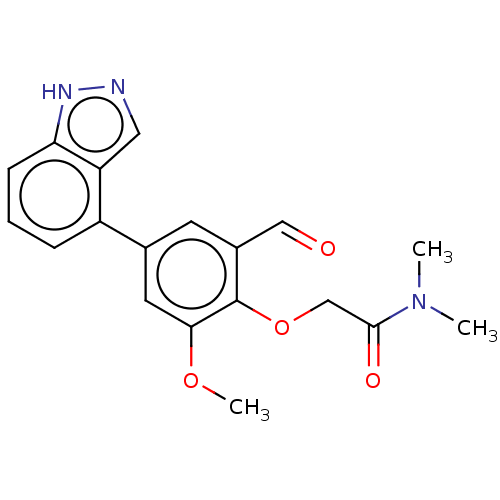
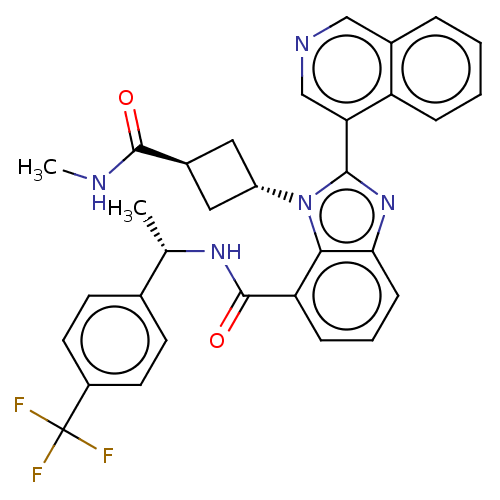
 3D Structure (crystal)
3D Structure (crystal)