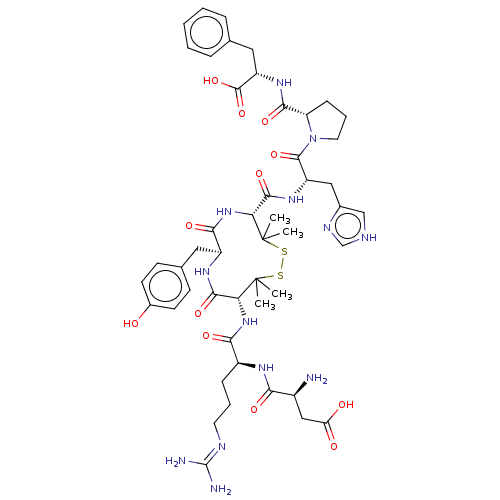
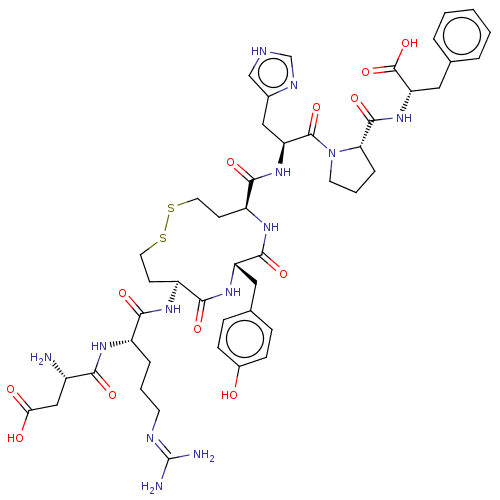
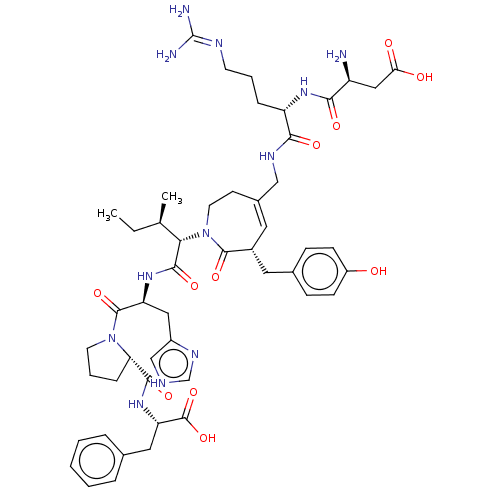
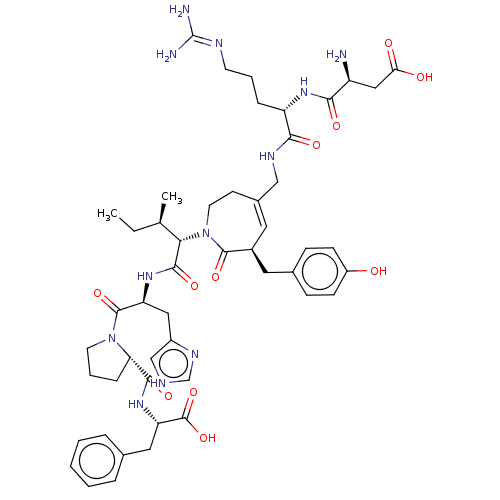
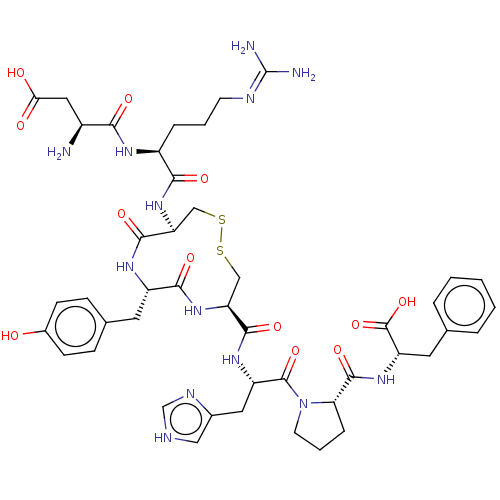
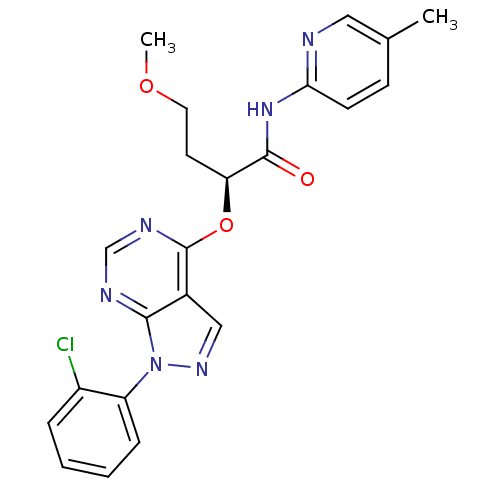
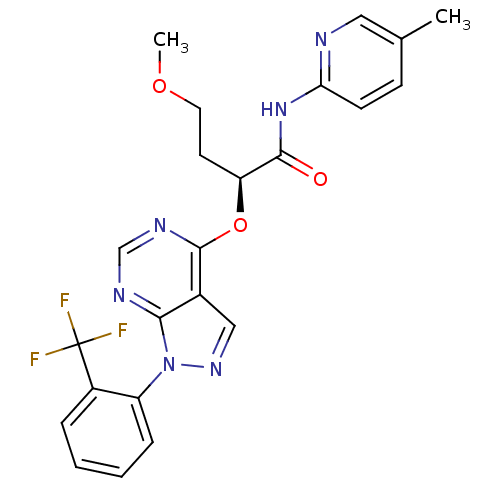
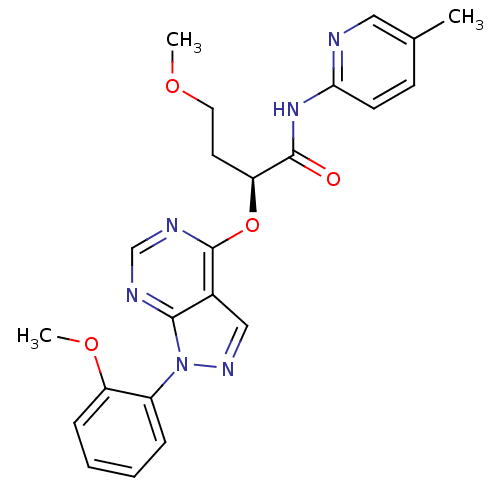
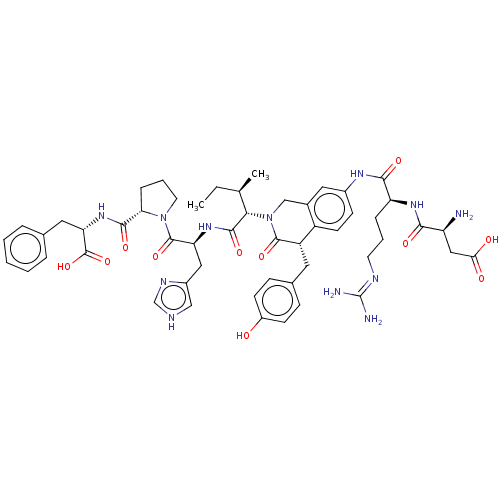
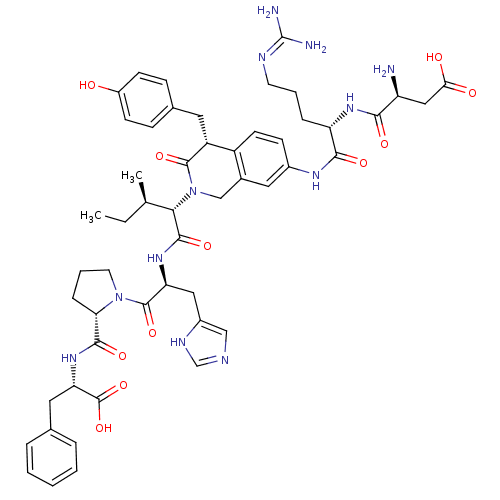
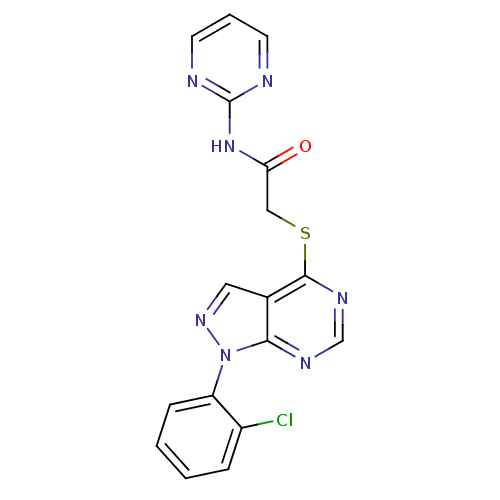
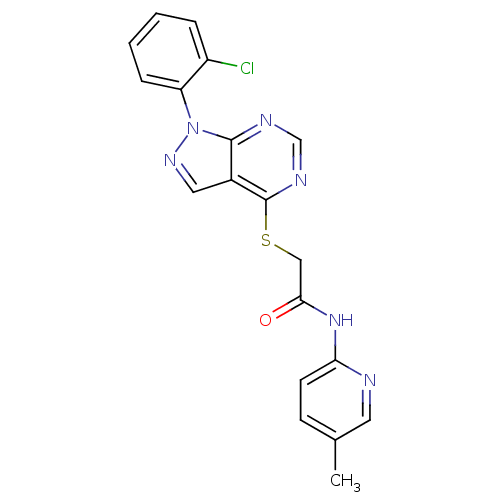
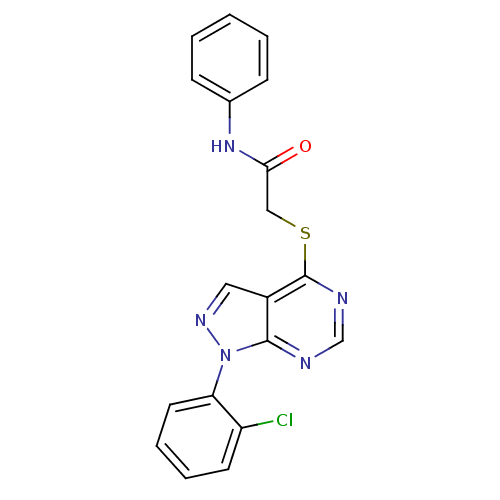
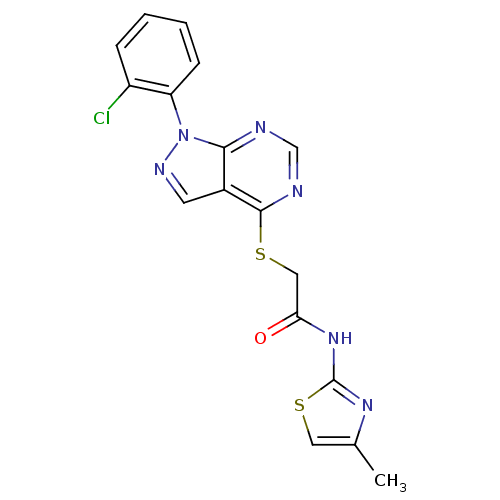
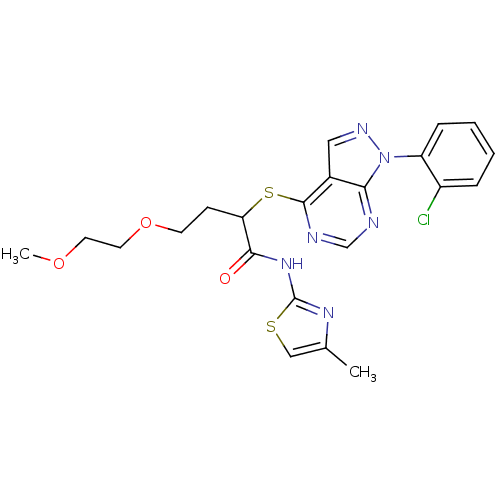
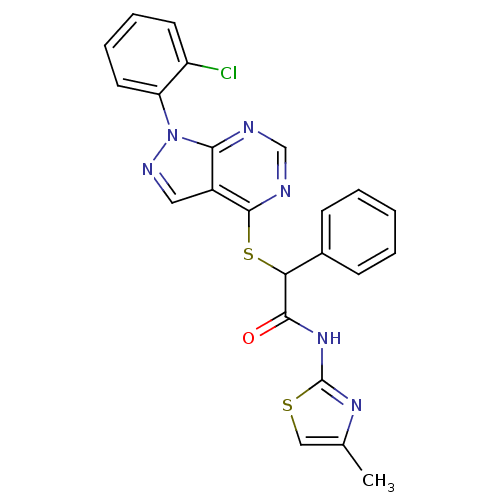
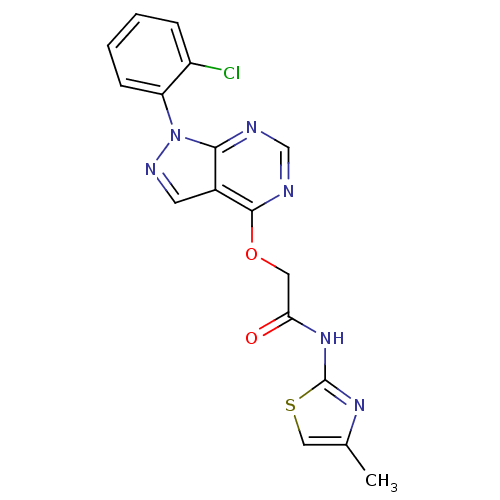
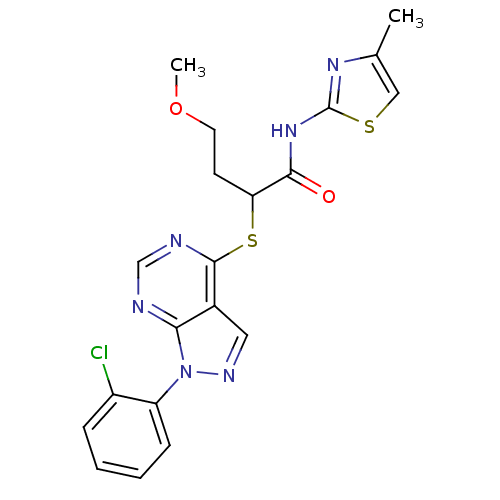
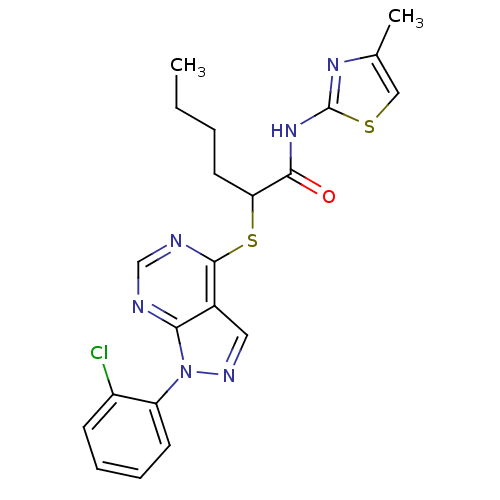
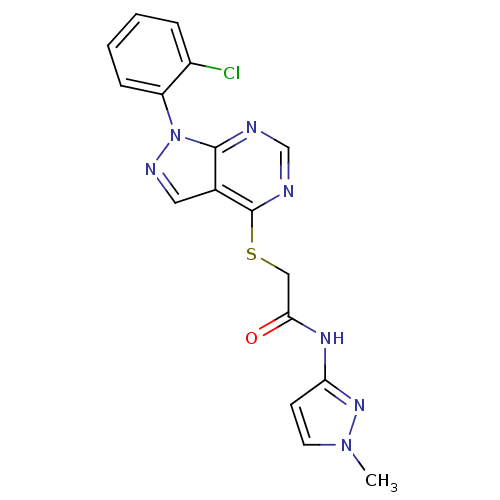
Affinity DataIC50: 0.900nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1)More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1)More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibitory concentration against specific binding of [125 I]Ang II to rat pituitary membranes (Angiotensin II receptor, type 1) in presence of 0.2% b...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibitory concentration against specific binding of [125 I]Ang II to rat pituitary membranes (Angiotensin II receptor, type 1) in presence of 0.2% b...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibitory concentration against specific binding of [125 I]Ang II to rat pituitary membranes (Angiotensin II receptor, type 1) in presence of 0.2% b...More data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibitory concentration against specific binding of [125 I]Ang II to rat uterine membranes (Angiotensin II receptor, type 1)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
AstraZeneca
Curated by ChEMBL
AstraZeneca
Curated by ChEMBL
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against [125 I]Ang II binding to rat pituitary membranes Angiotensin II receptor type 1 without 0.2% bovine serum albuminMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 501nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 2.51E+3nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 3.16E+3nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 1.58E+3nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 126nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 316nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 200nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 50.1nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 63.1nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 126nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 794nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 631nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair
Affinity DataEC50: 1.58E+4nMAssay Description:Activation of human glucokinaseMore data for this Ligand-Target Pair