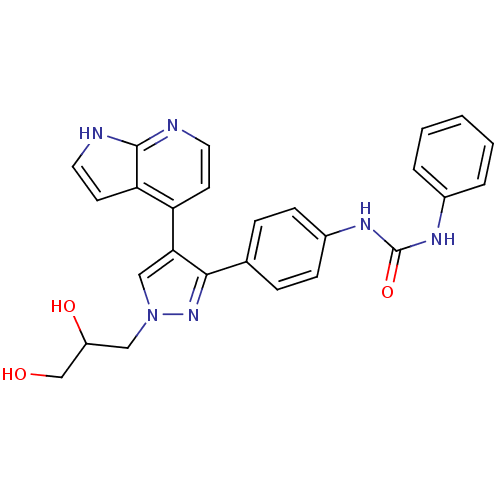
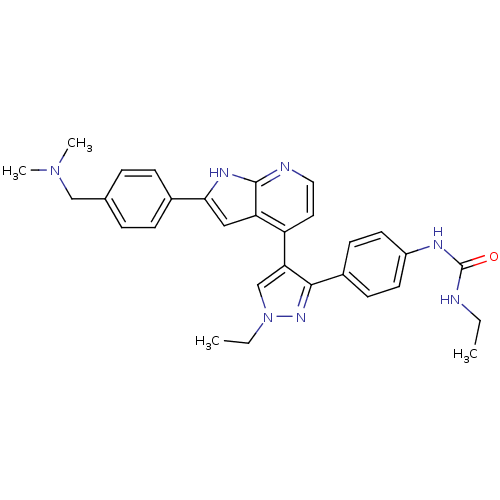
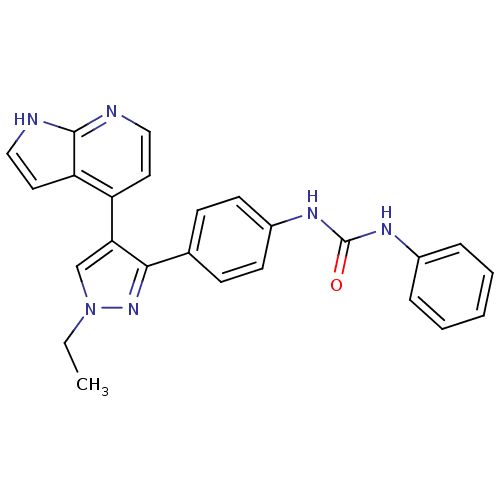
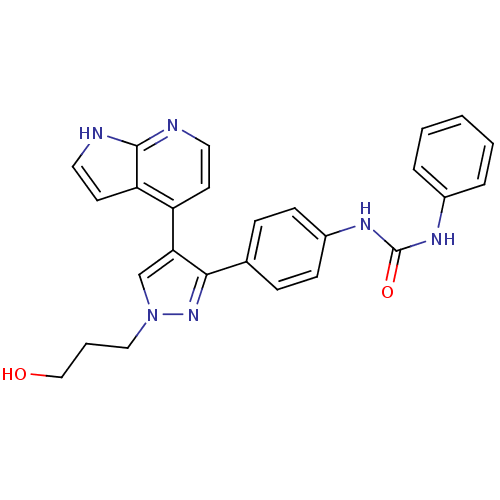
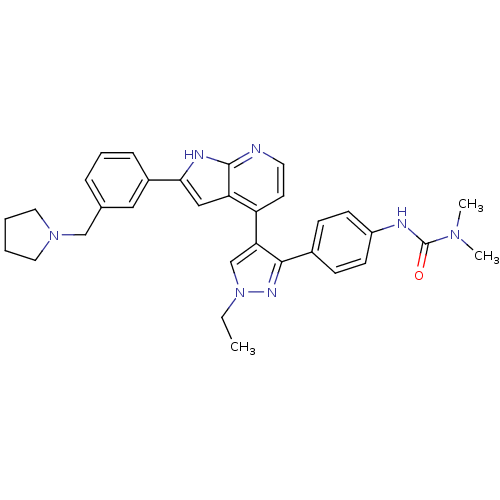
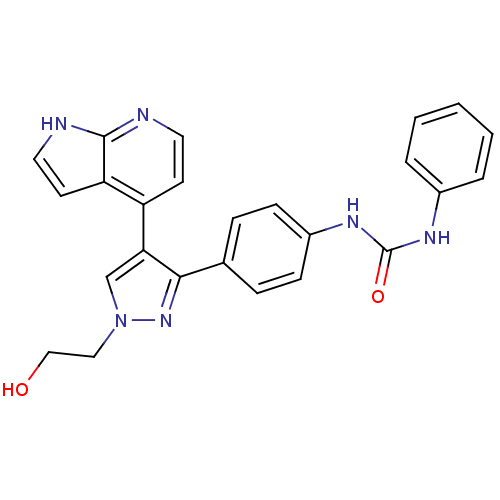
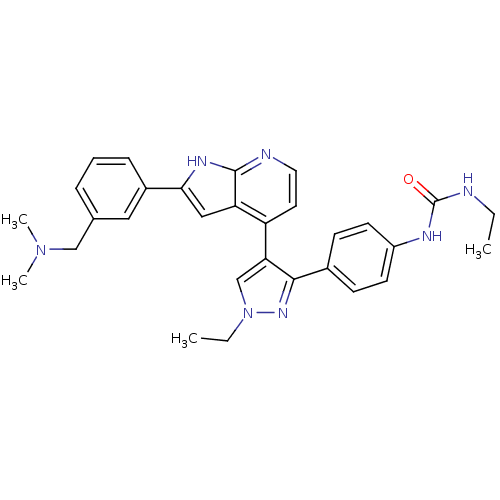
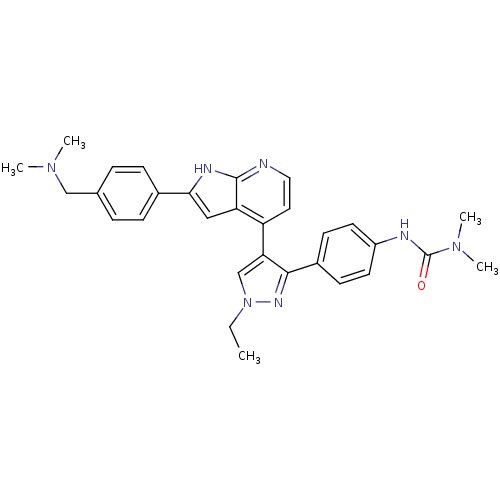
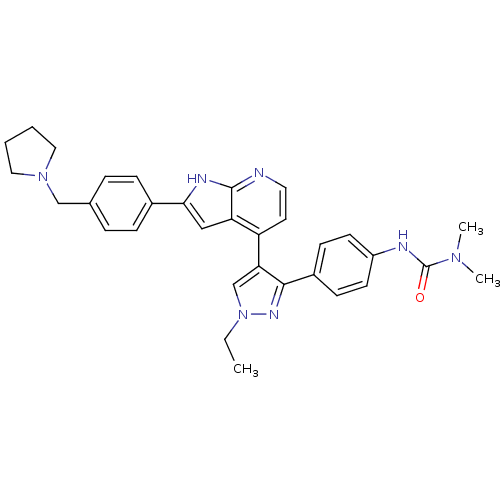
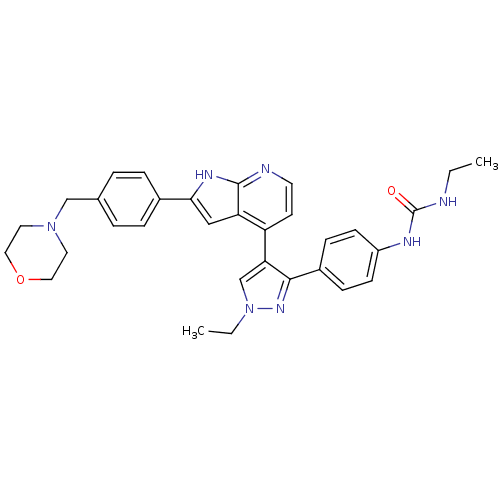
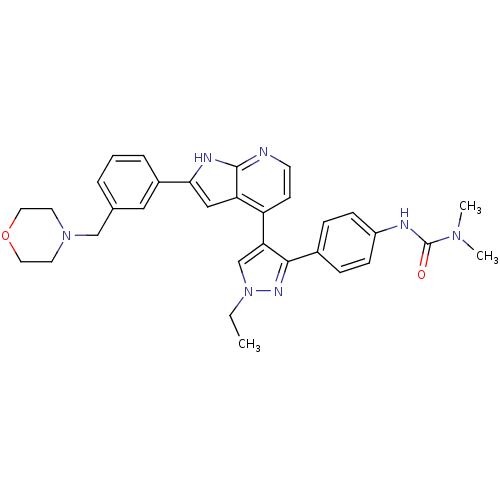
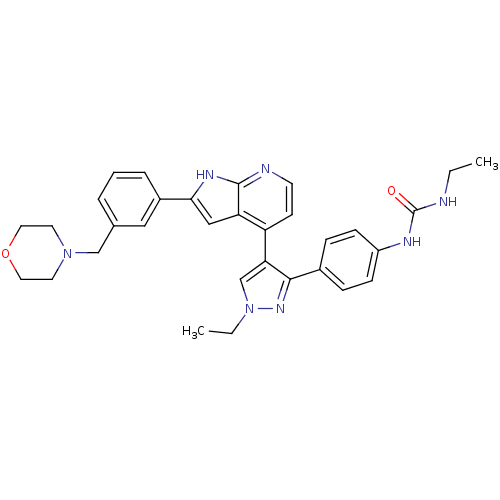
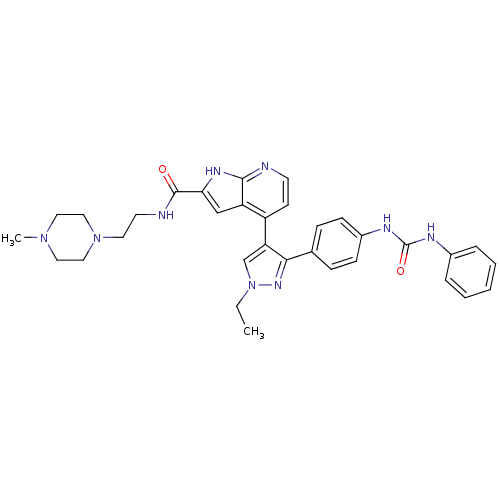
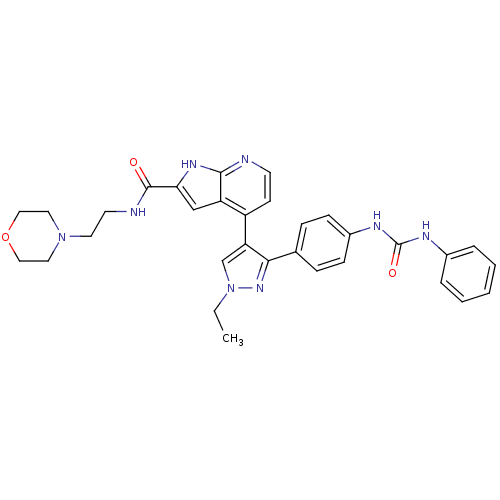
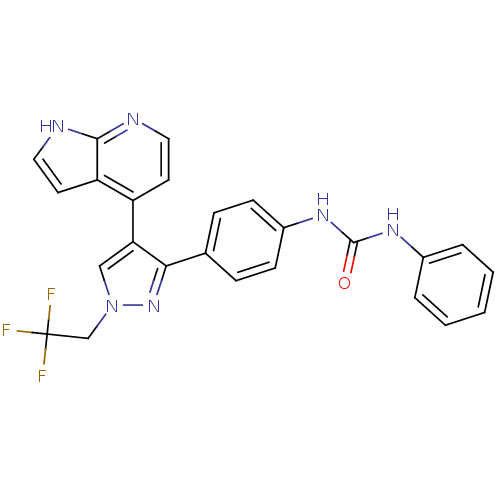
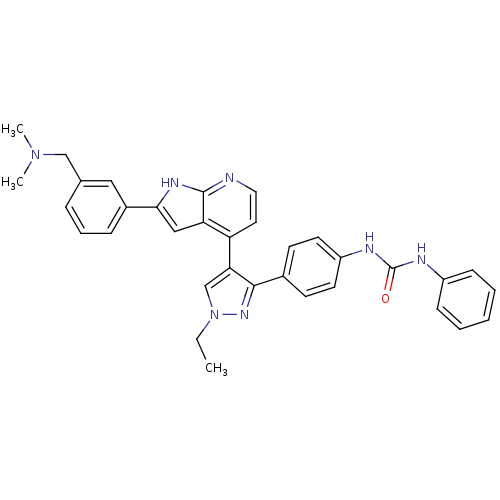
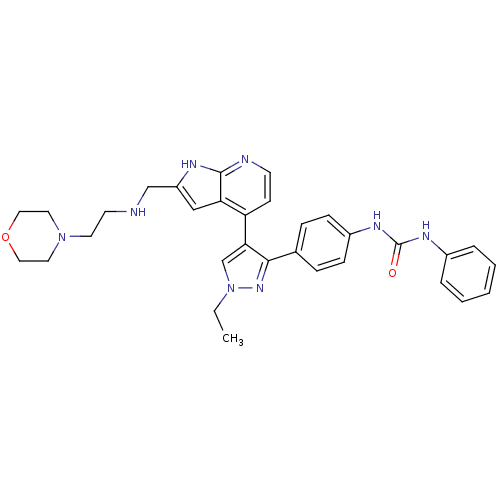
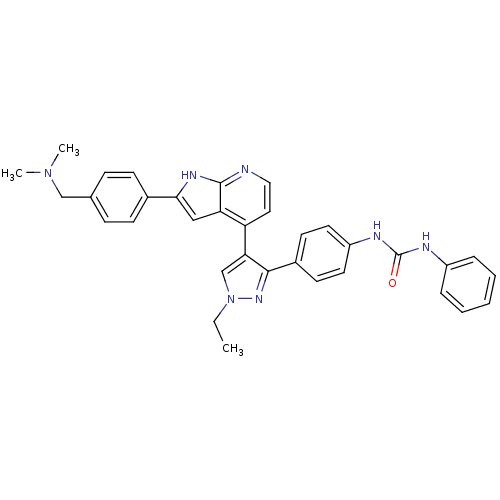
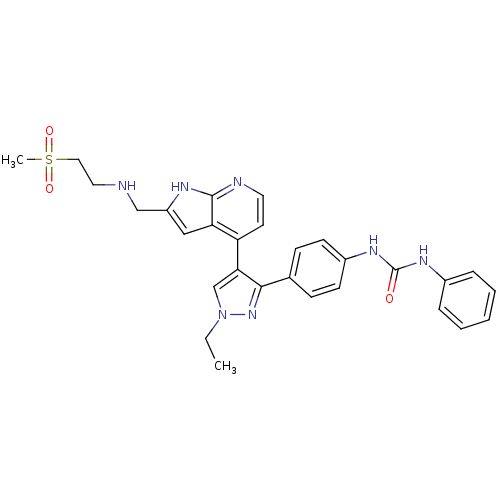
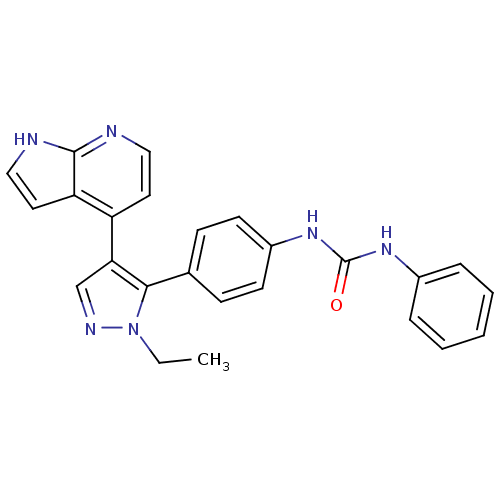
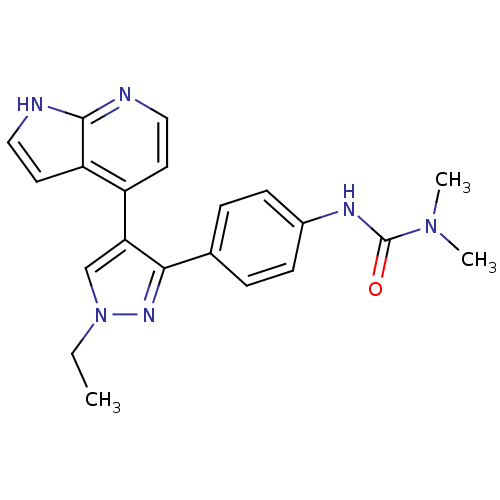
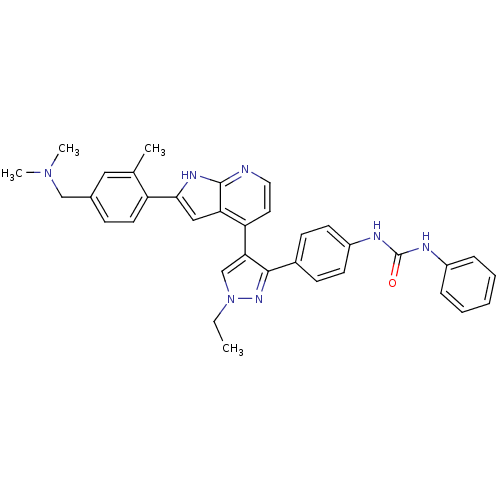
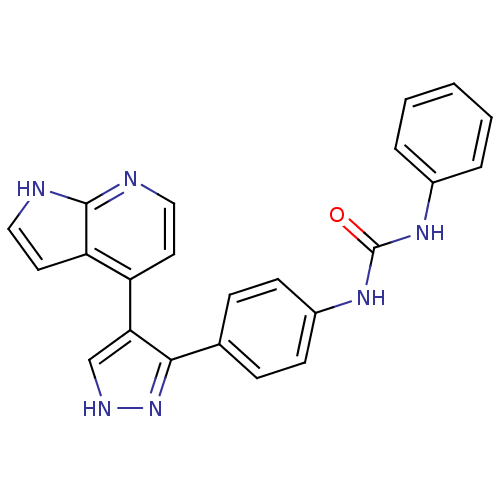
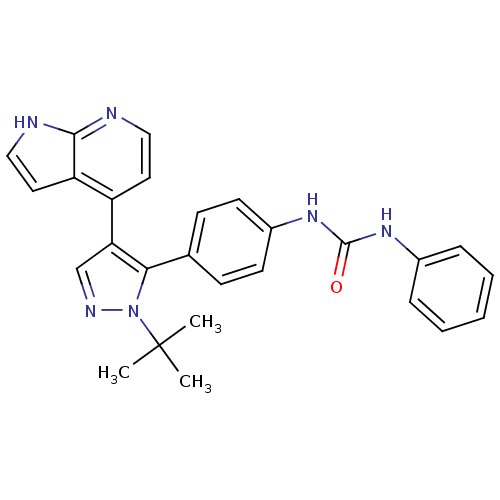
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of human Aurora B ATP binding site by rapid dilution methodMore data for this Ligand-Target Pair
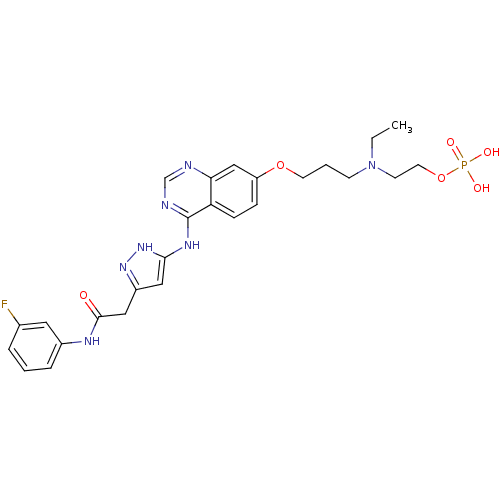
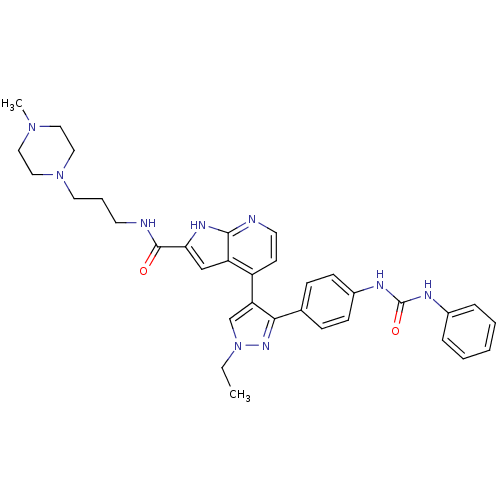
Affinity DataKi: 0.380nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
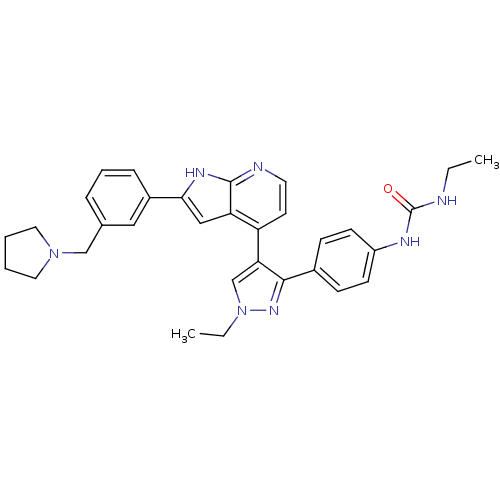
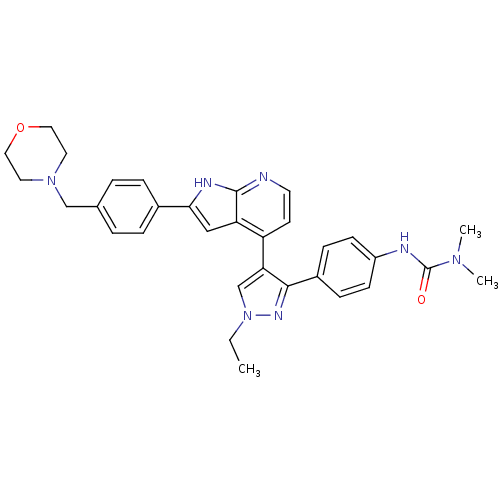
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora AMore data for this Ligand-Target Pair
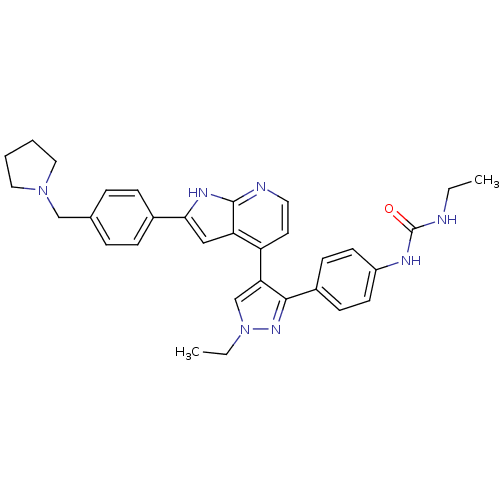
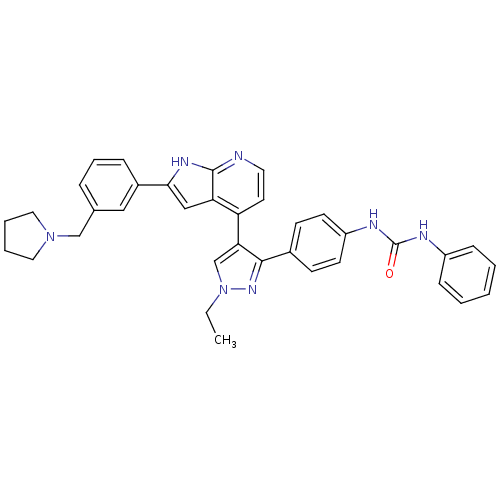
Affinity DataKi: 1.5nMAssay Description:Competitive inhibition of human Aurora C ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Competitive inhibition of Aurora C ATP binding siteMore data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:Competitive inhibition of human Aurora A ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Competitive inhibition of Aurora B ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Competitive inhibition of Aurora C ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of aurora B assessed as inhibition of histone H3 phosphorylation by ELISA based cellular mechanistic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Competitive inhibition of Aurora C ATP binding siteMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human Aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of human Aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of aurora B assessed as inhibition of histone H3 phosphorylation by ELISA based cellular mechanistic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of human Aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human Aurora B by rapid dilution methodMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of human Aurora AMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human Aurora AMore data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)