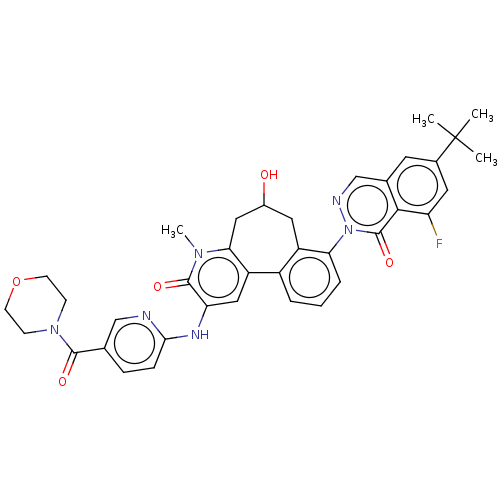
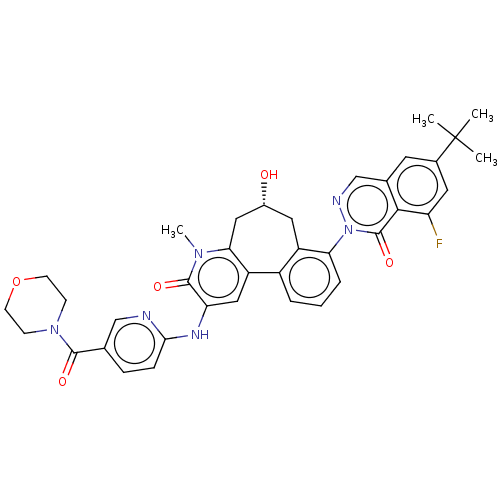
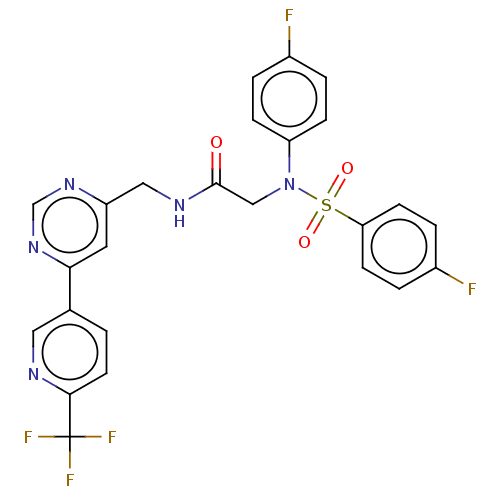
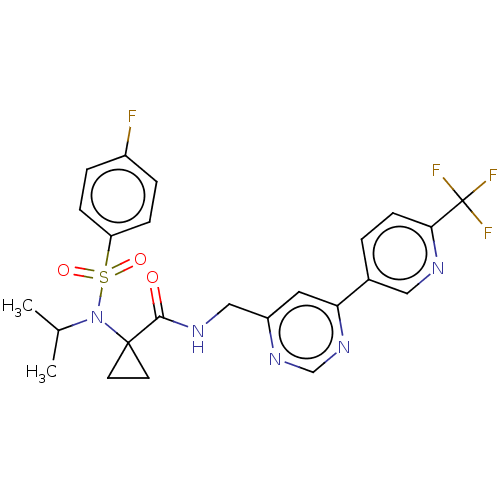
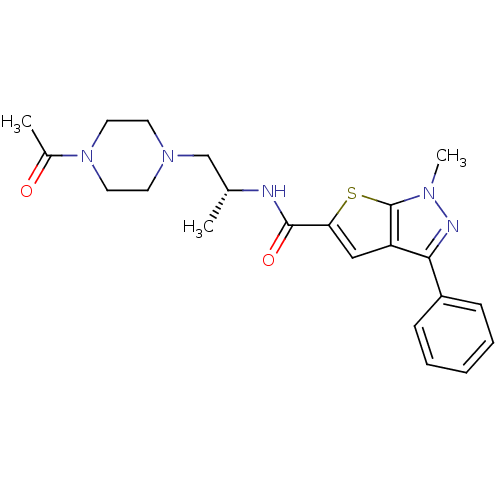
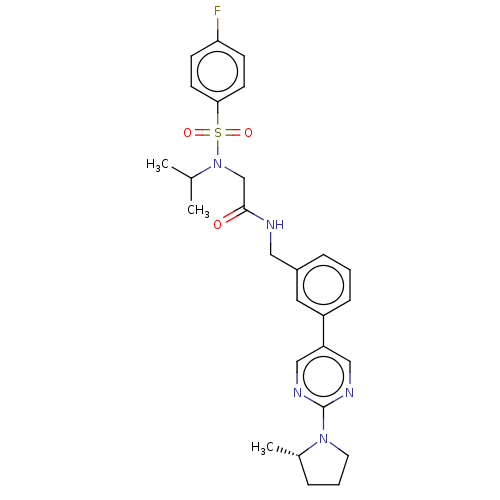
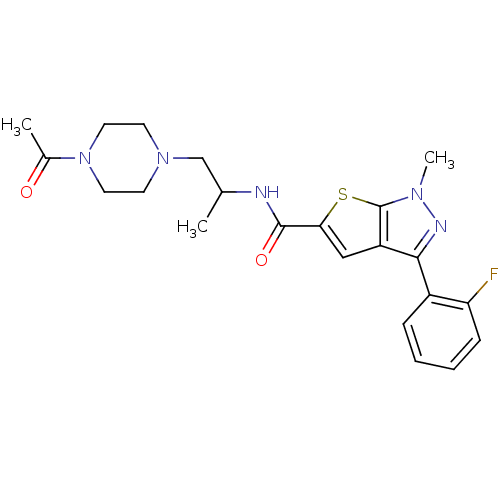
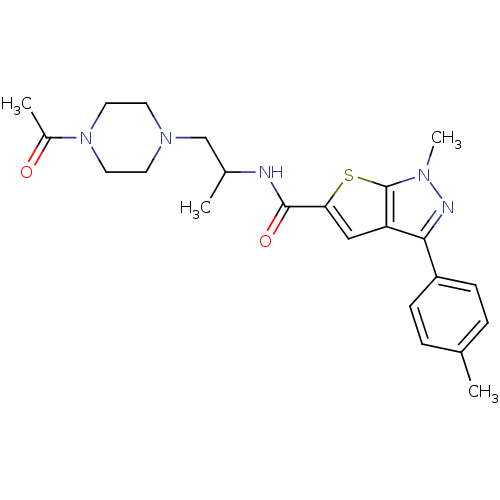
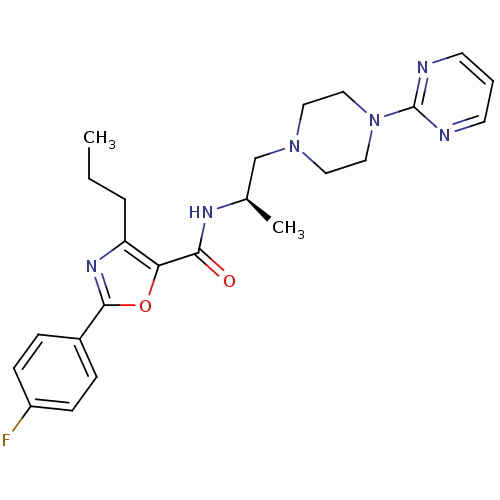
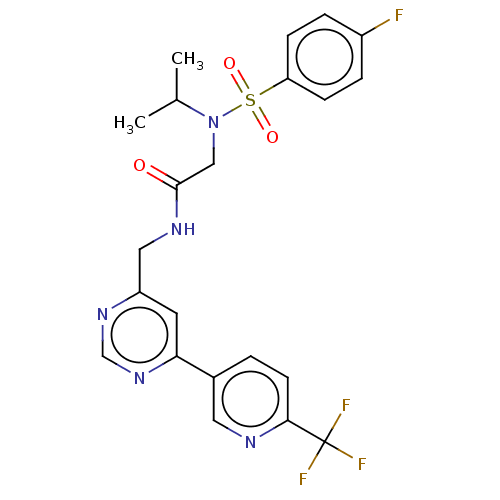
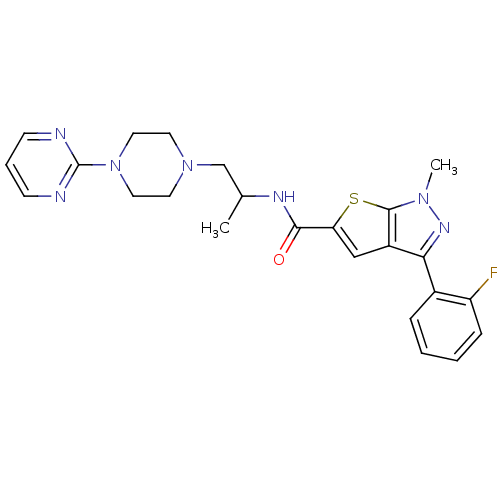
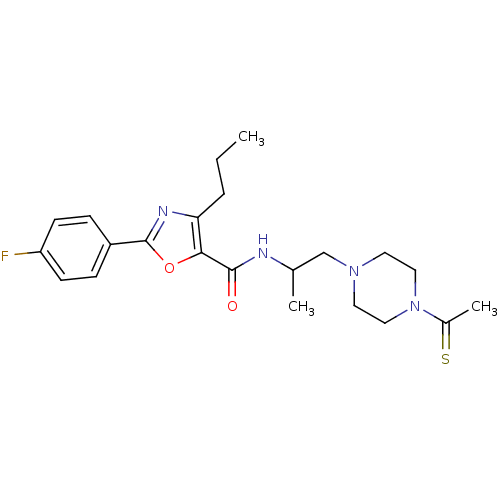
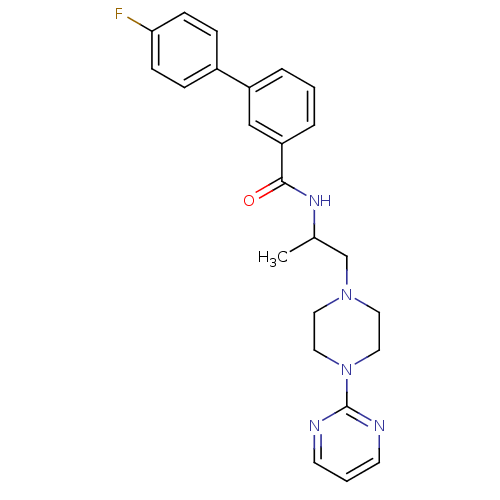
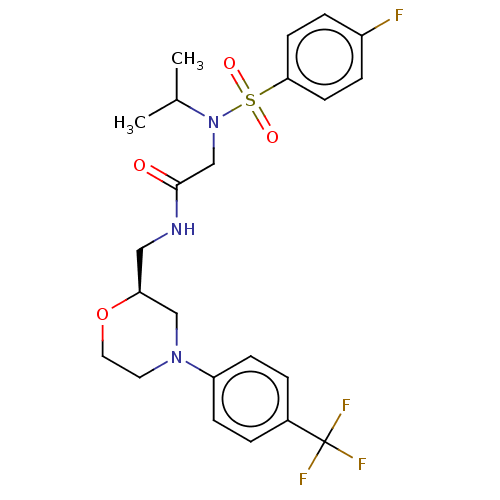
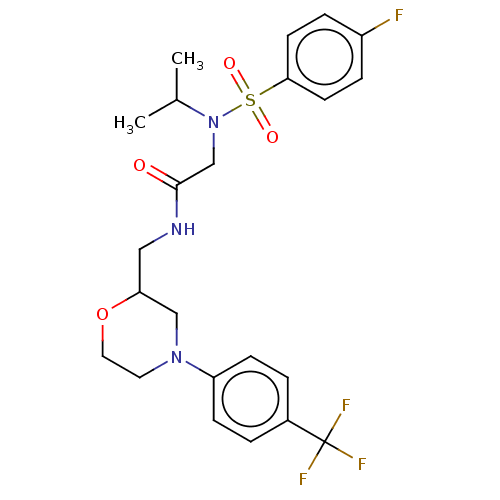
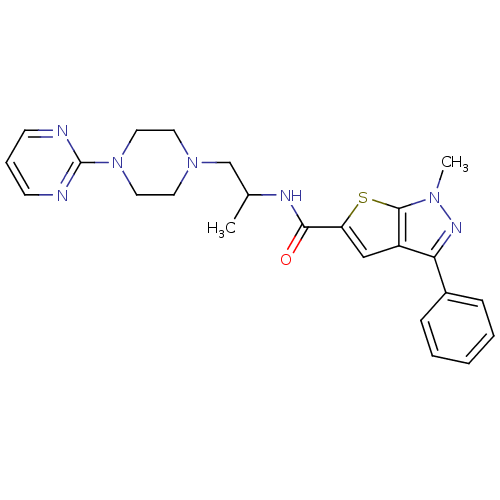
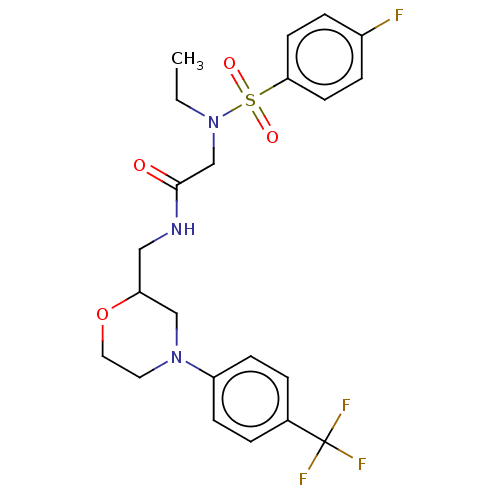
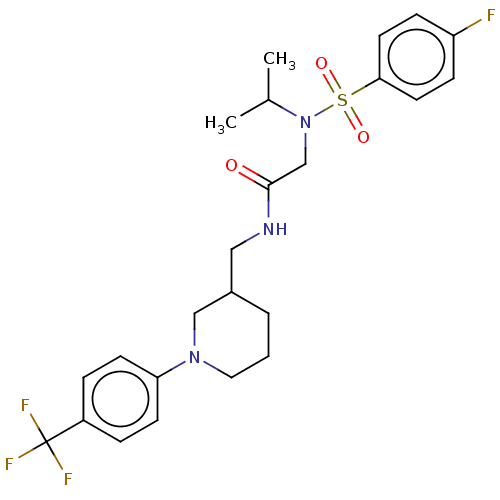
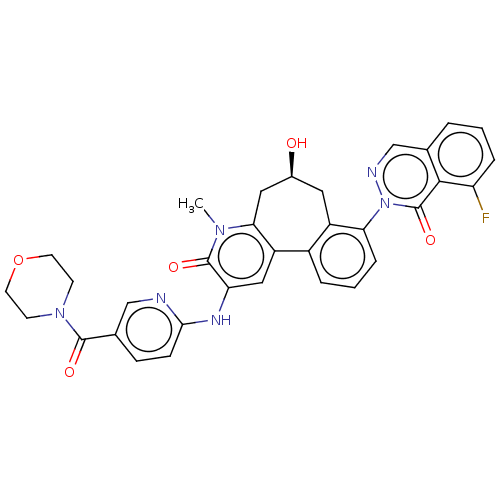
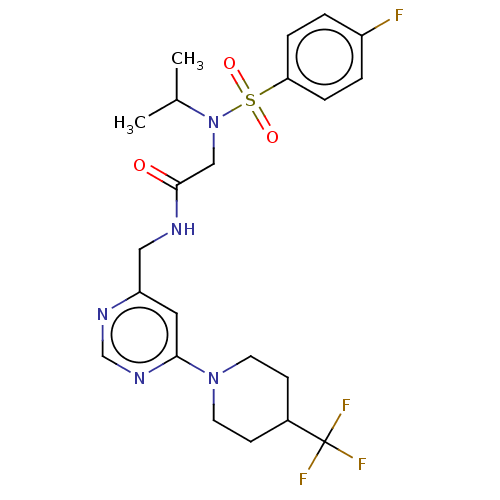
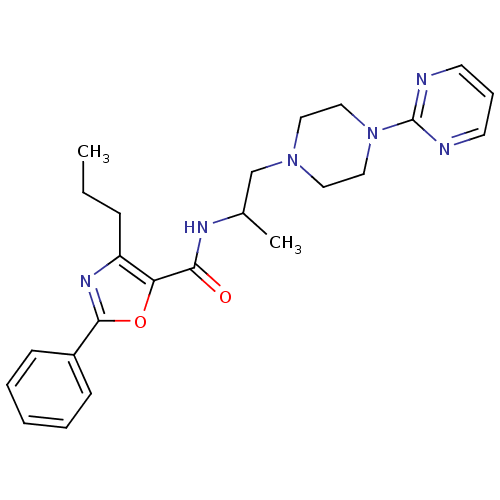
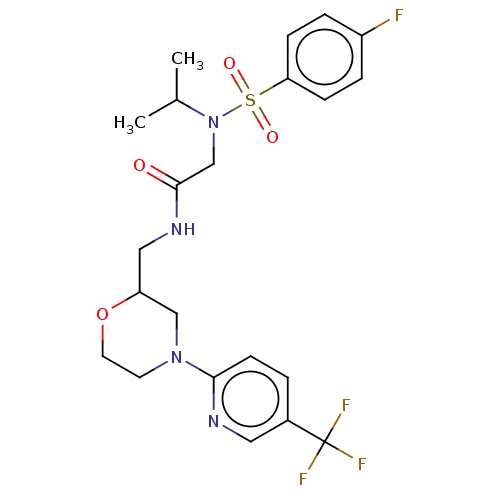
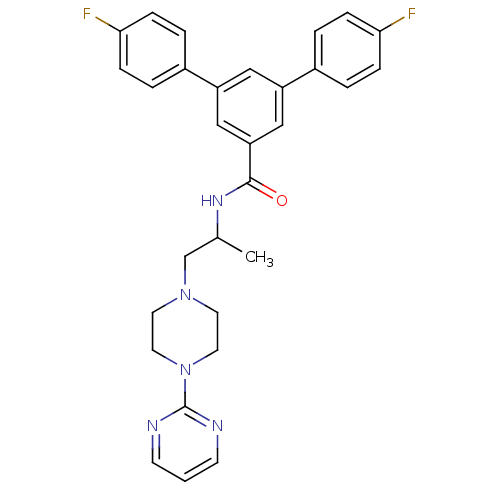
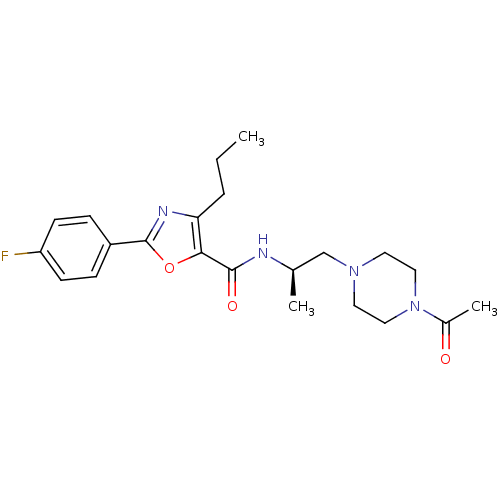
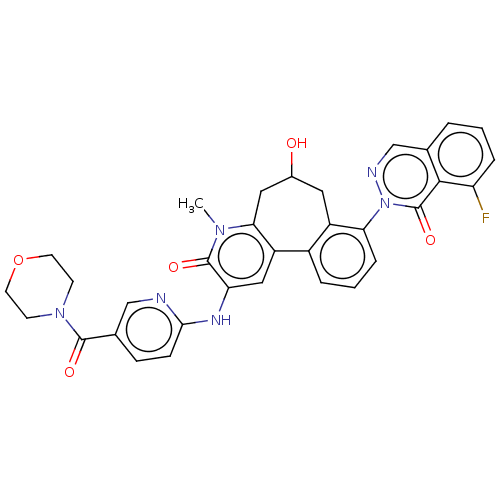
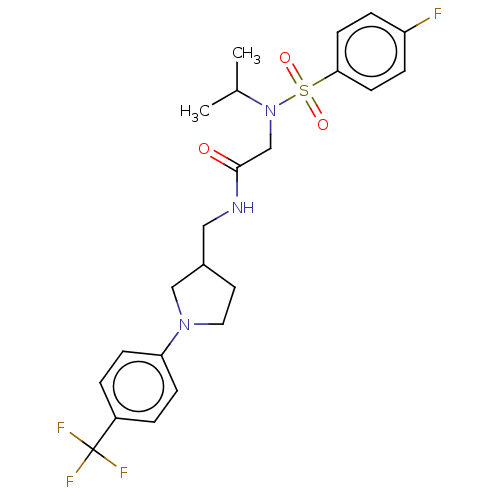
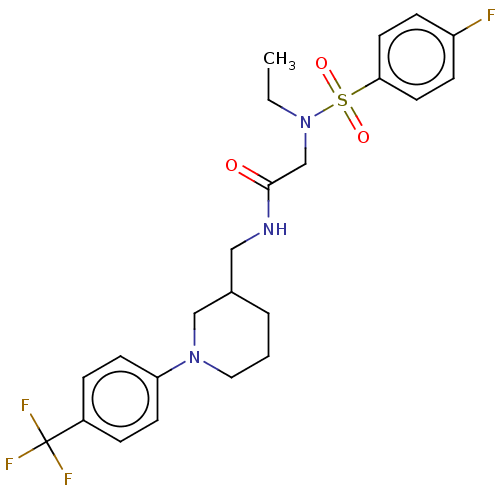
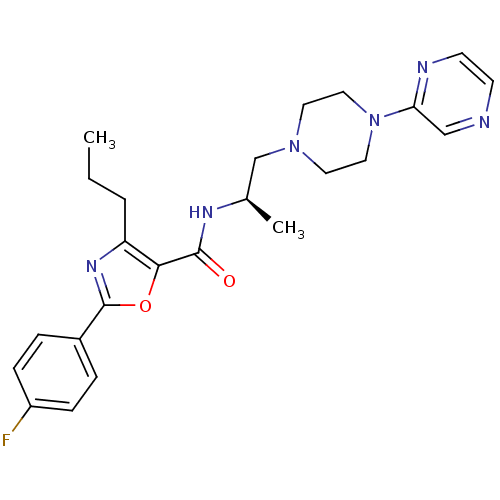
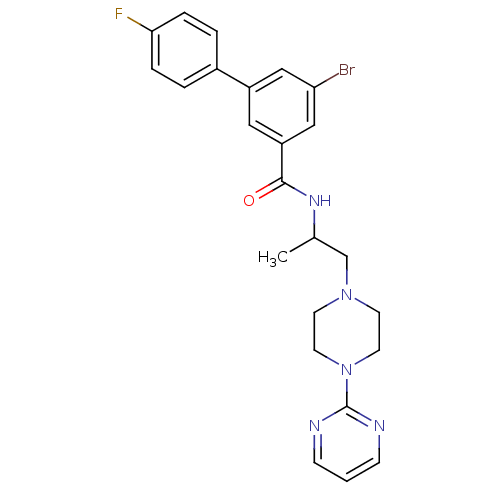
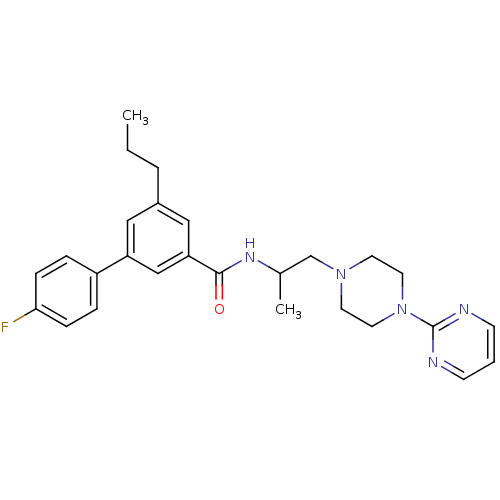
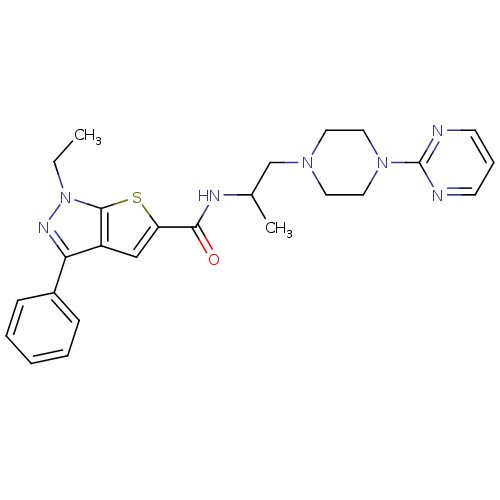
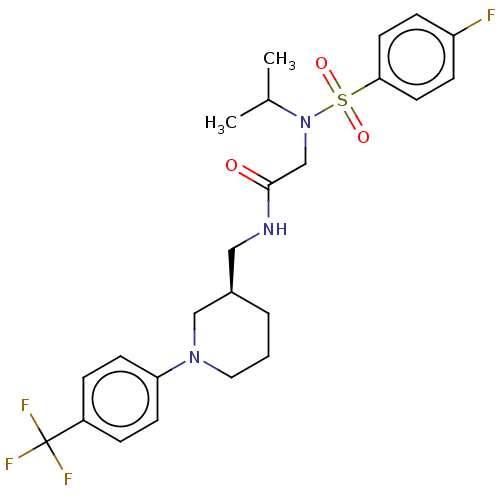
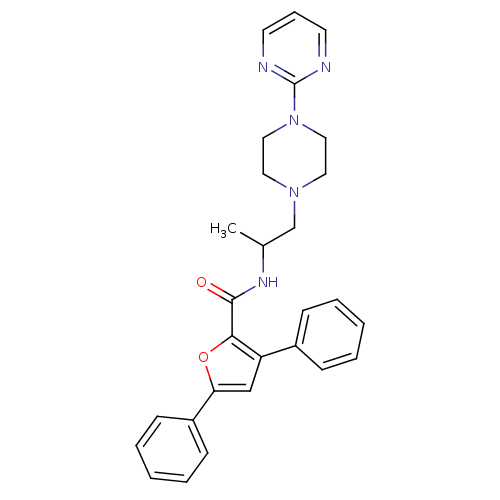
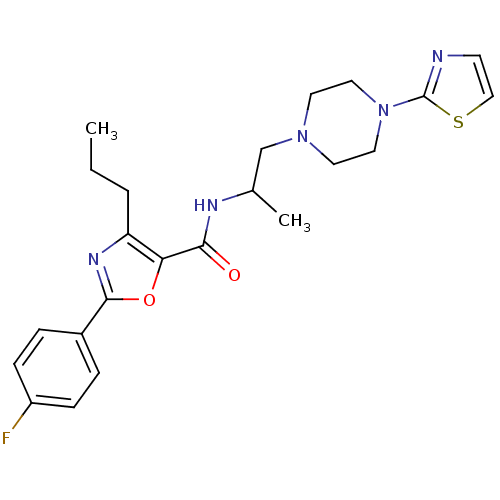
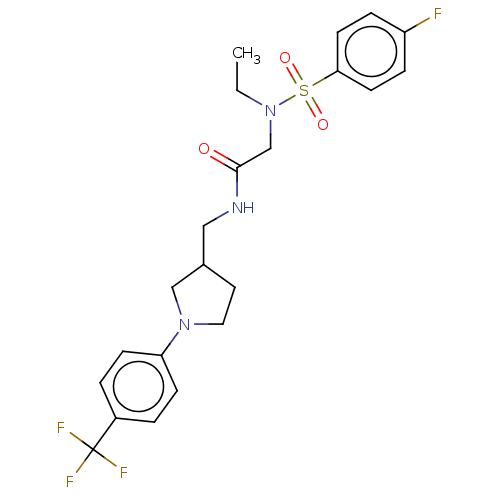
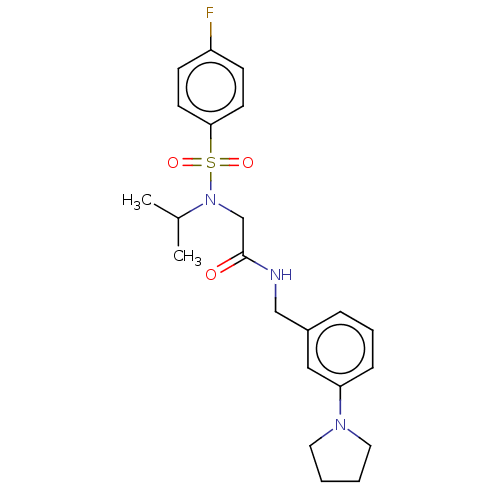
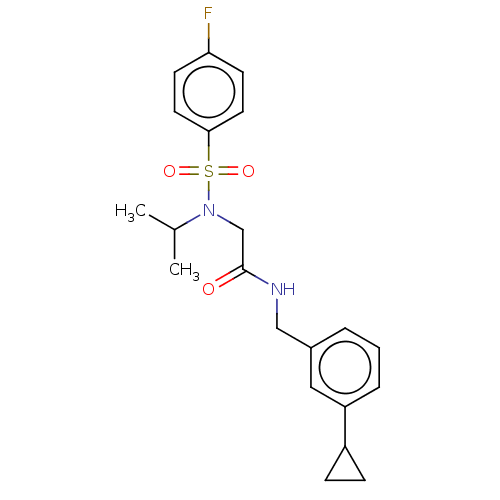
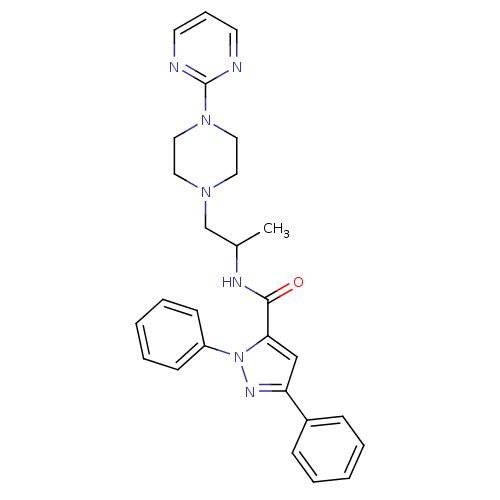
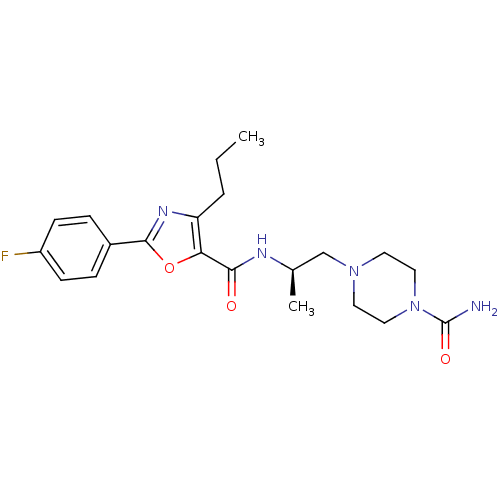
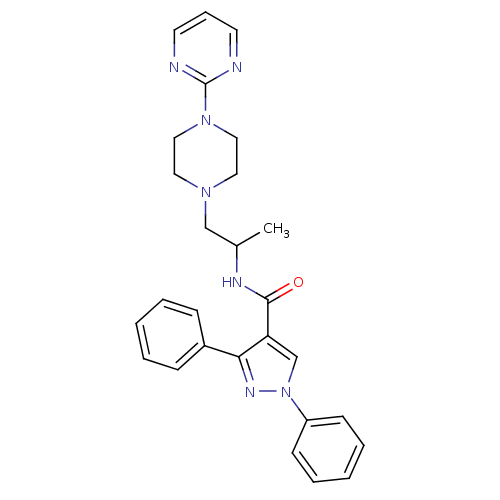
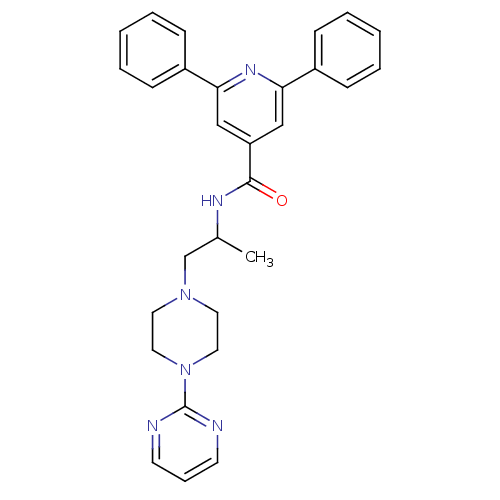
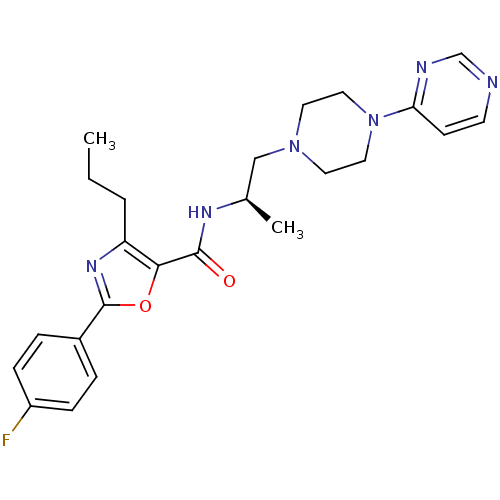
Affinity DataIC50: 0.820nMpH: 7.2Assay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
Affinity DataIC50: 0.910nMpH: 7.2Assay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMpH: 7.2Assay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.2Assay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 9nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 13nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 29nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
Affinity DataIC50: 31.6nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 38nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
Affinity DataIC50: 39.8nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 39.8nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 50.1nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 55nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 58nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
Affinity DataIC50: 63.1nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 151nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 193nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 226nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 233nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
Affinity DataIC50: 235nMpH: 7.2Assay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 251nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
Affinity DataIC50: 251nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 307nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 385nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Antagonist activity at human P2X3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 414nMpH: 7.2Assay Description:The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 425nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 494nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
Affinity DataIC50: 501nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 501nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 501nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 501nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 504nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
Affinity DataIC50: 631nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 631nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 634nMT: 2°CAssay Description:Compound plates were resuspended with 100 ul of HBSS/20 mM HEPES/0.005% BSA buffer (Compound Buffer): column 1A-H: buffer/DMSO (bk); column 2A-H: AP-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 883nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hoffmann-La Roche Inc.
US Patent
Hoffmann-La Roche Inc.
US Patent
Affinity DataIC50: 1.00E+3nMT: 2°CAssay Description:IC50s (effective concentration) of compounds on the human TRPA1 channel were determined using a Hamamatsu FDSS fluorescence plate reader. CHO cells e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Antagonist activity at rat P2X3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair

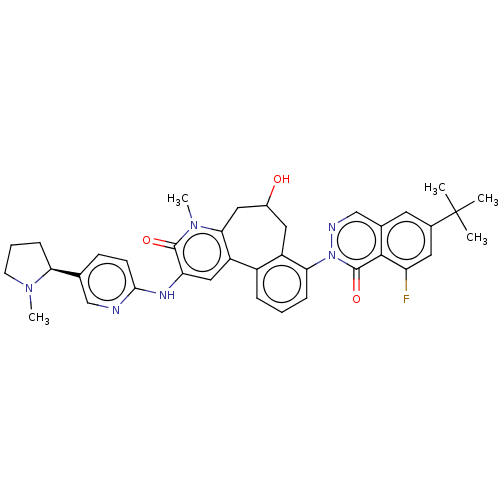
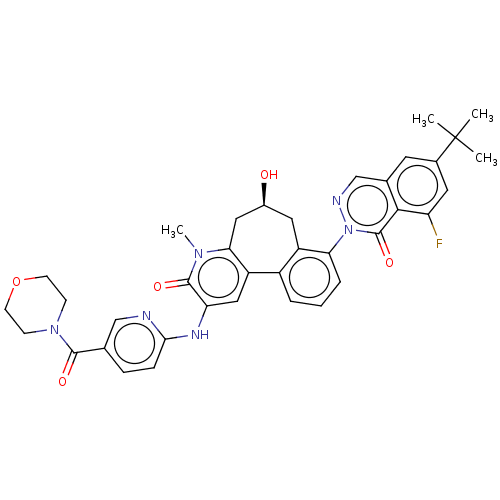
 3D Structure (crystal)
3D Structure (crystal)