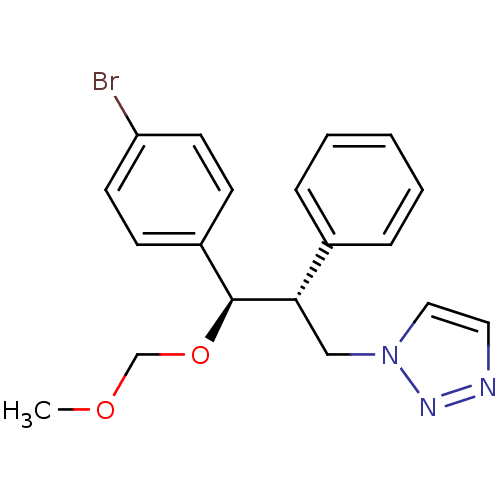
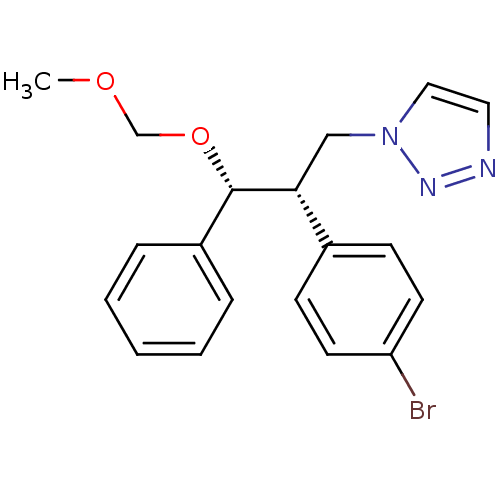
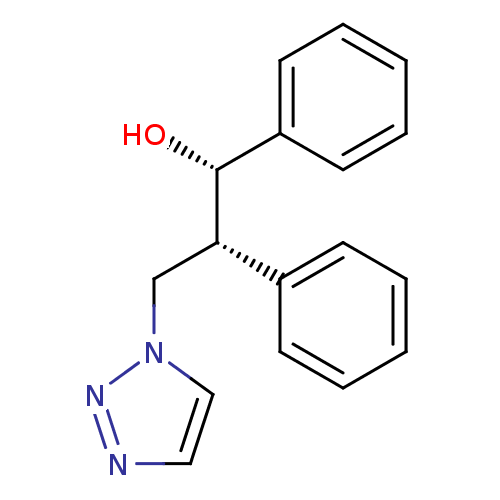
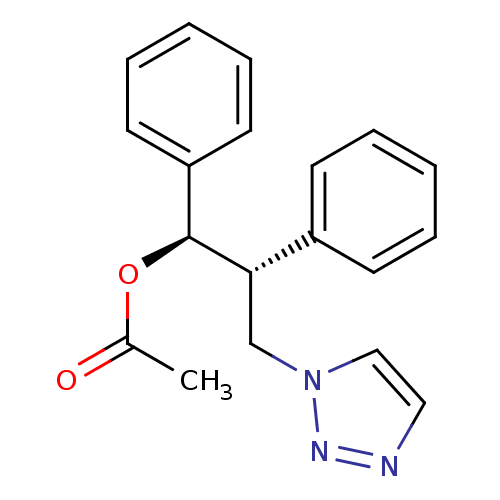
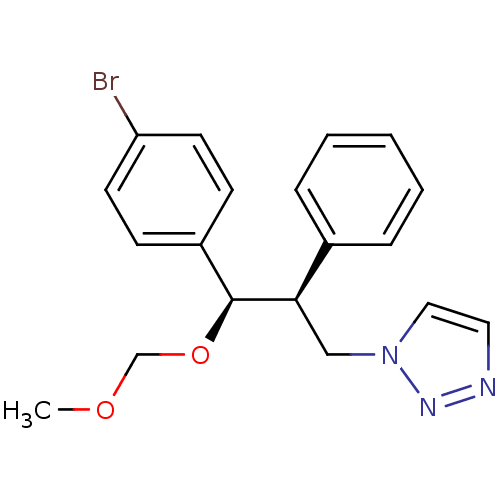
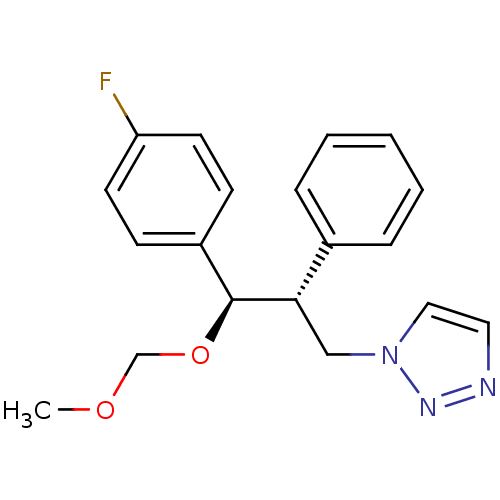
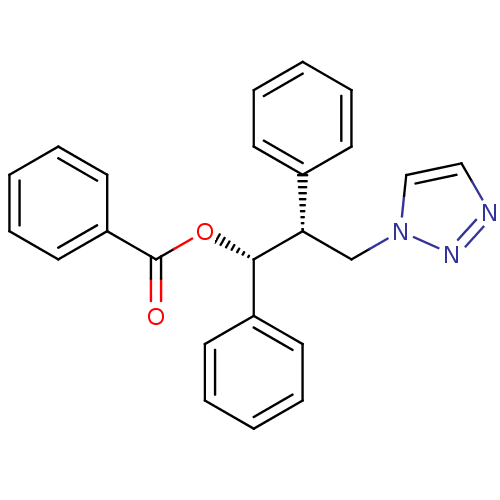
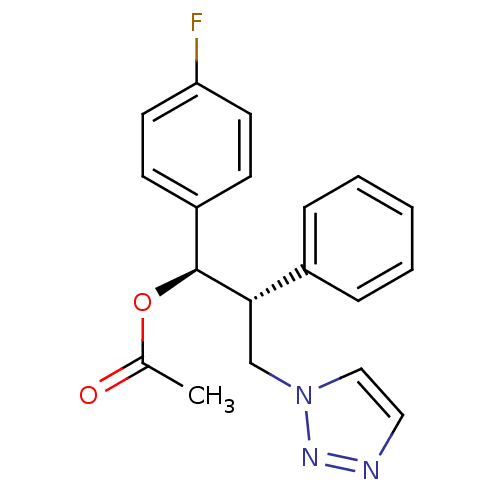
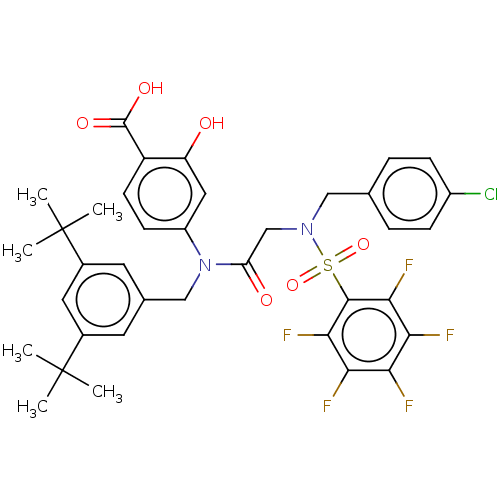
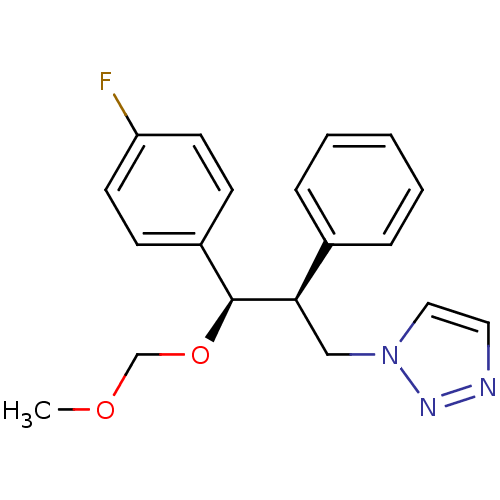
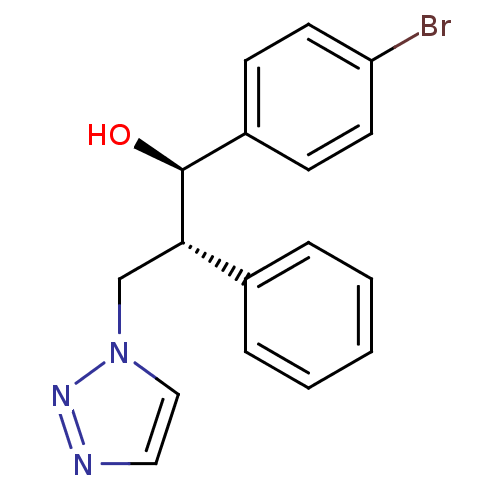
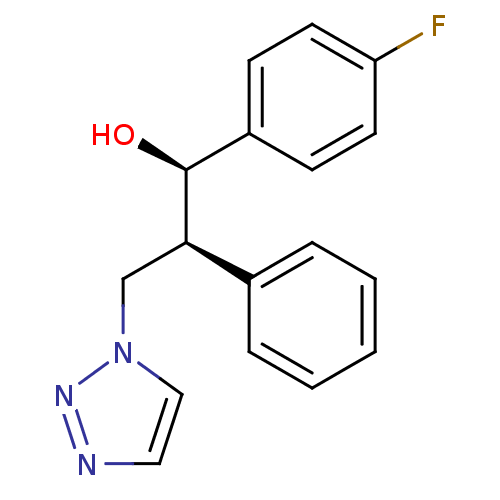
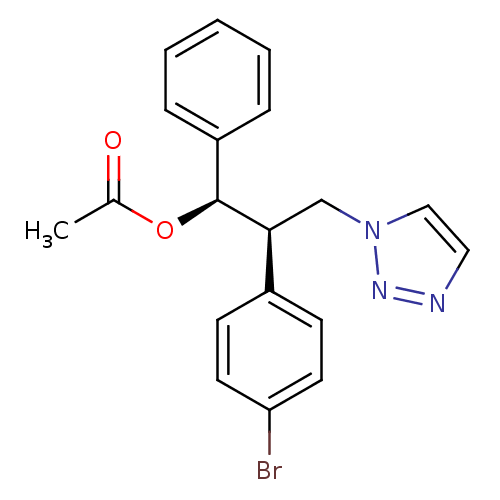
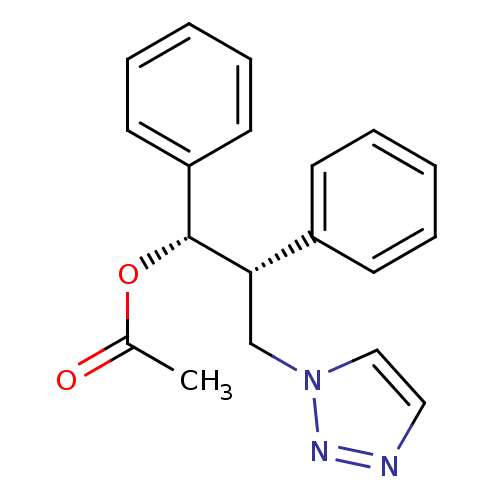
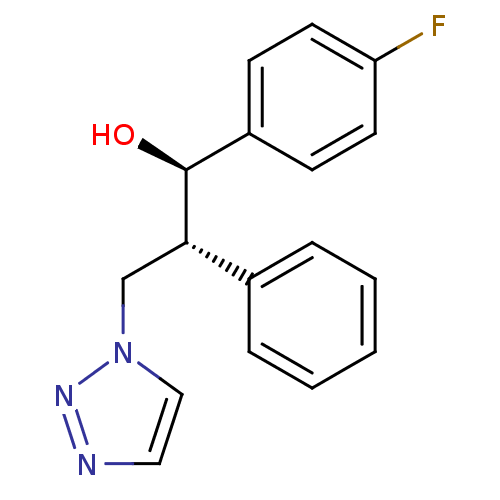
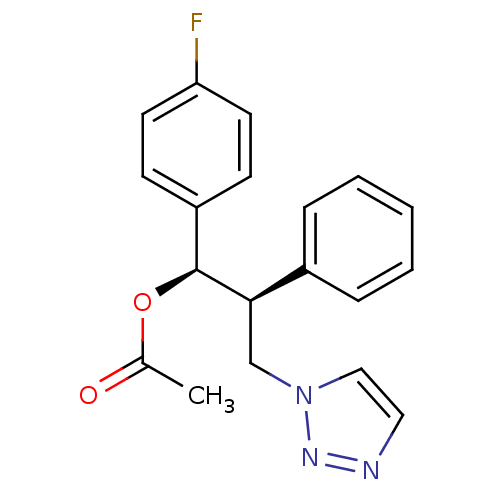
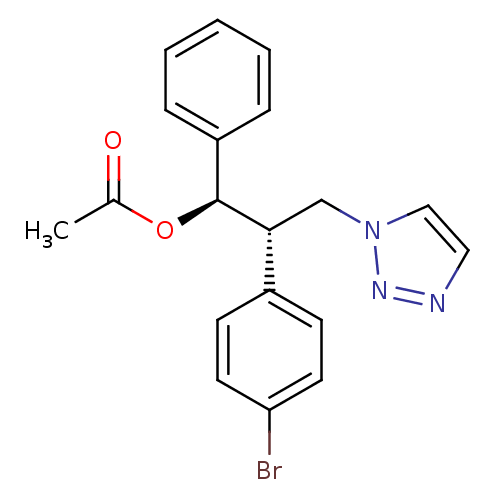
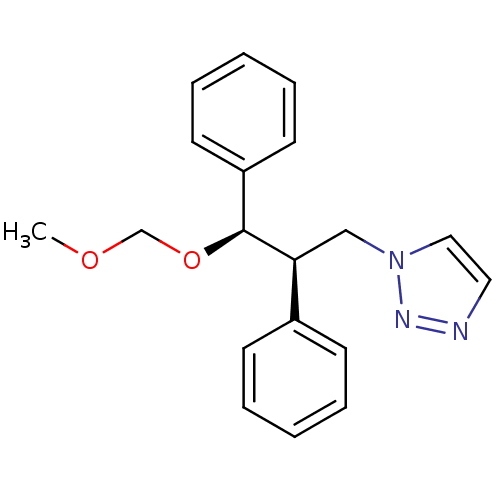
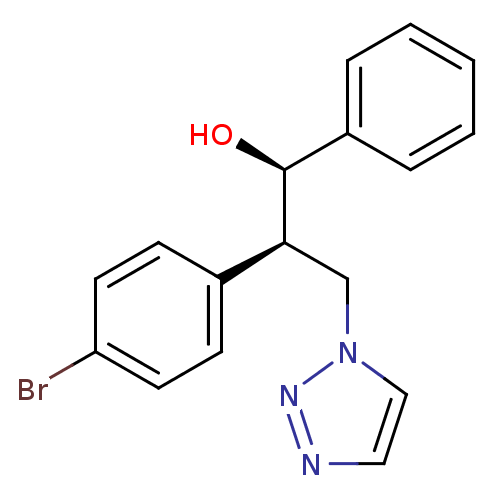
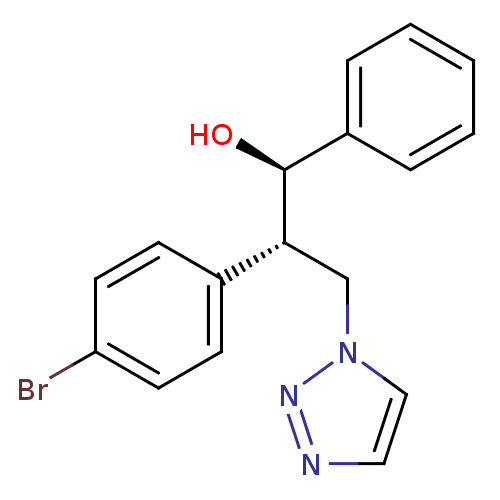
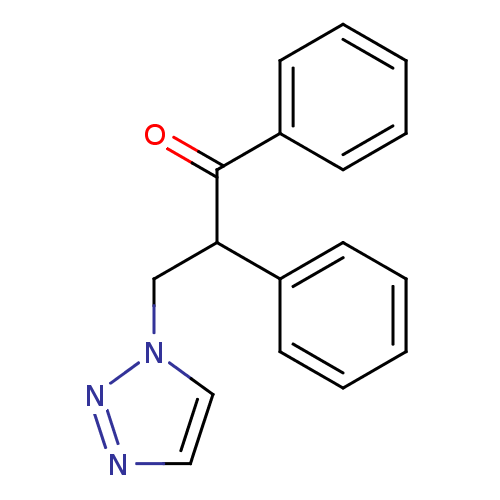
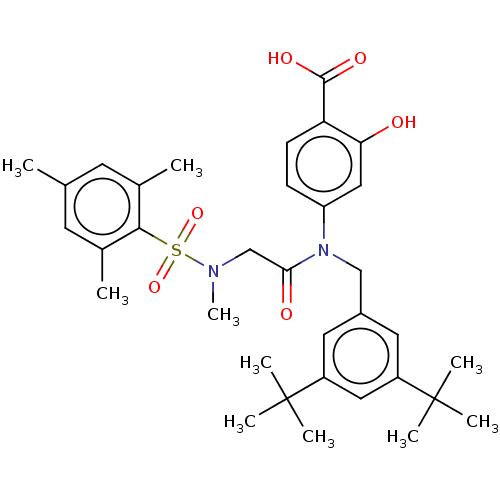
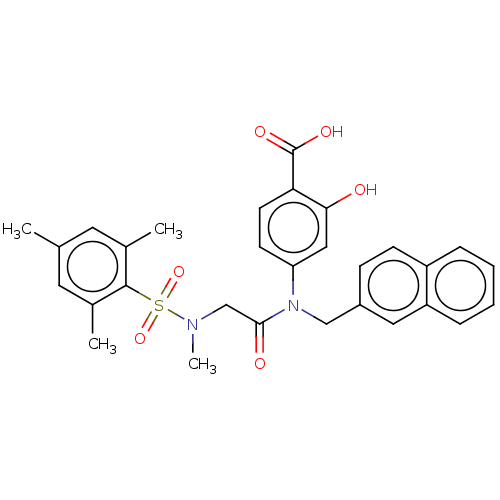
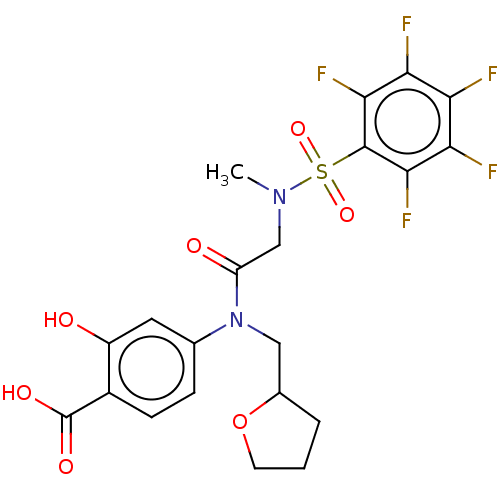
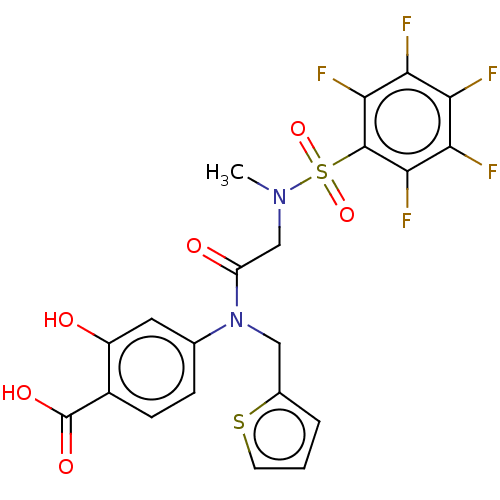
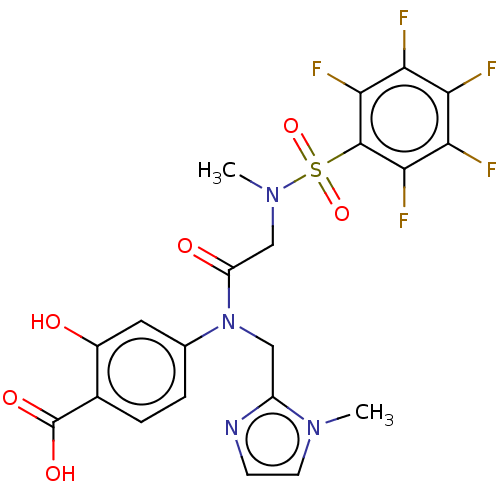
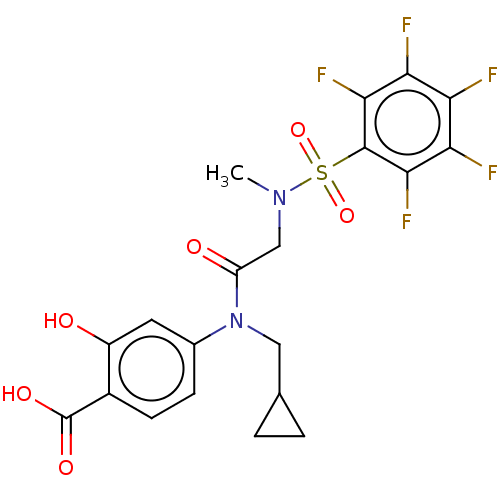
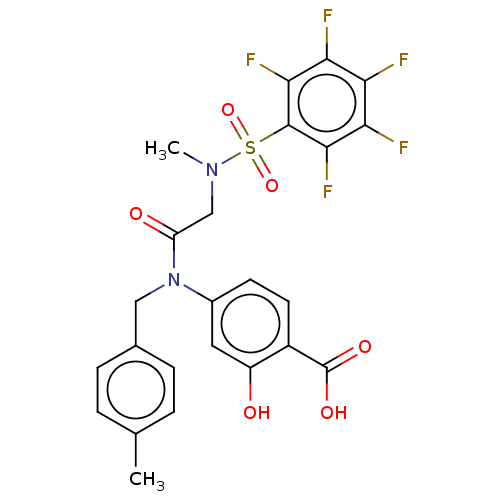
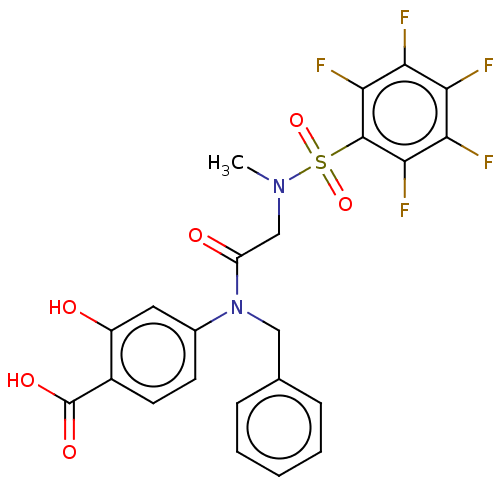
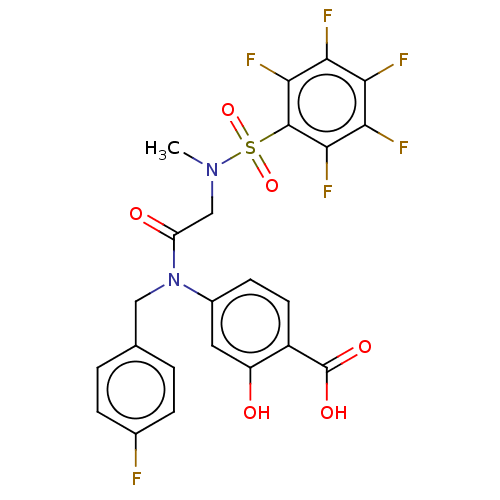
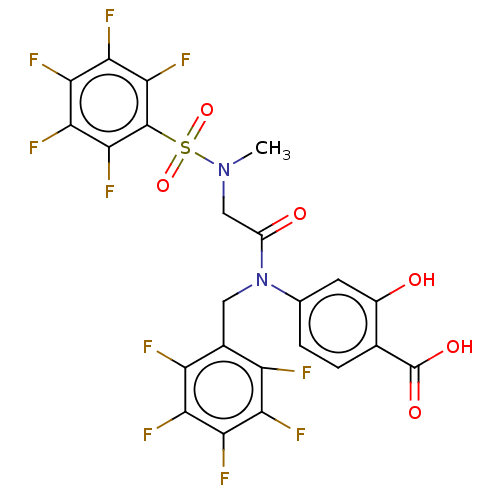
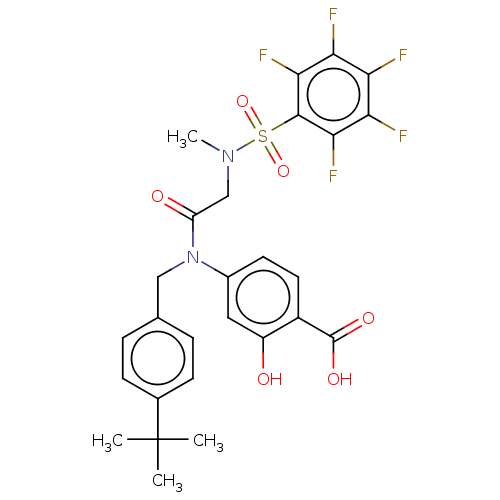
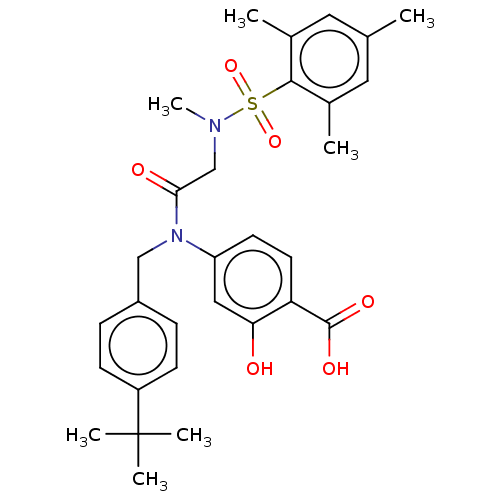
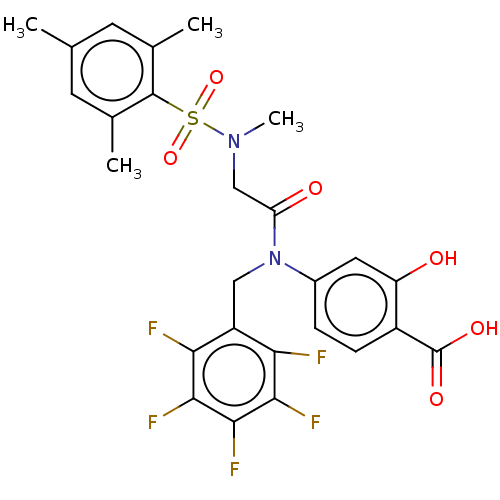
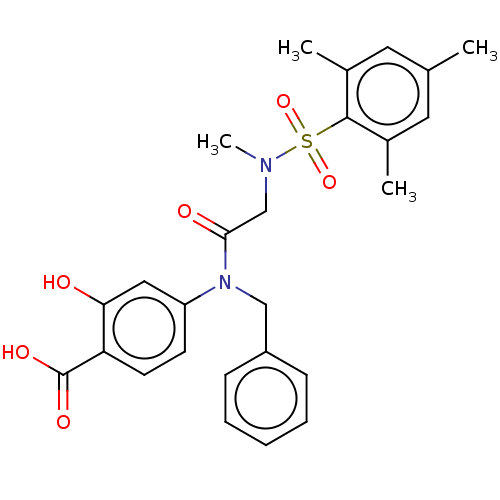
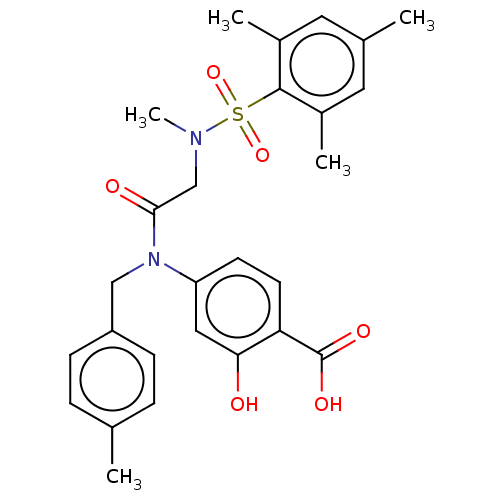
Affinity DataKi: 20nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 111nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 146nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 237nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 398nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 647nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
Affinity DataKi: 820nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 841nMAssay Description:Inhibition of recombinant human aromatase using O-dibenzylfluorescein benzyl ester as substrate by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human recombinant aromatase assessed as conversion of O-dibenzylfluorescein benzyl ester substrate to fluorescein byproduct by fluorome...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 4.77E+3nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.37E+3nMAssay Description:Inhibition of CYP1A1More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 6.62E+3nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 1.15E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 1.24E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.09E+4nMAssay Description:Inhibition of CYP1A1More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair