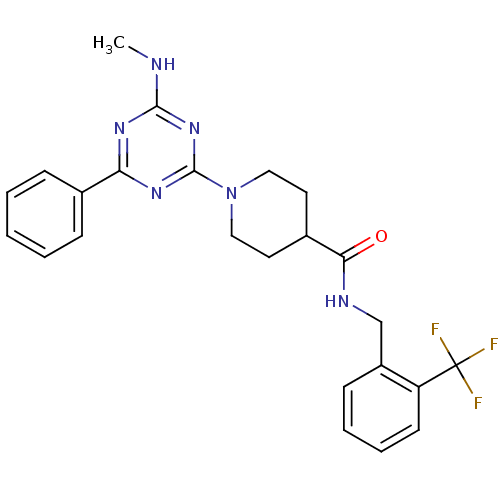
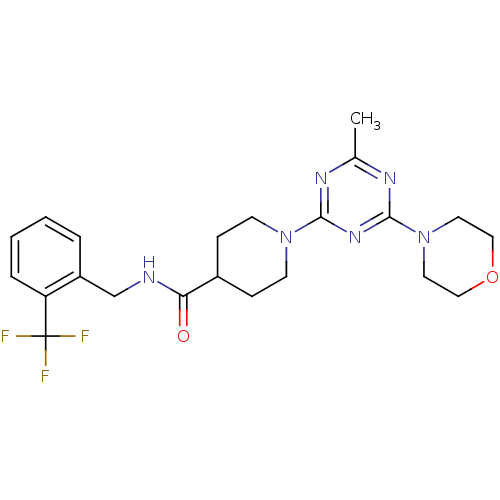
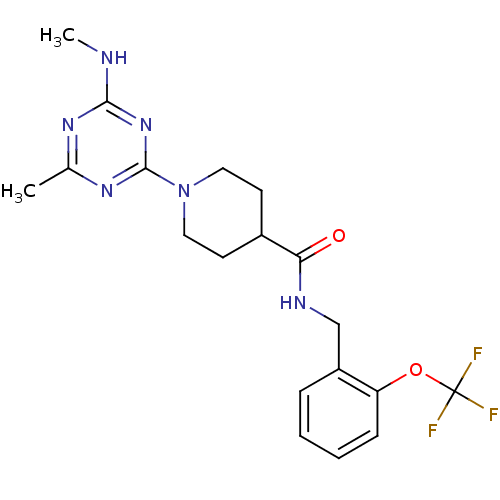
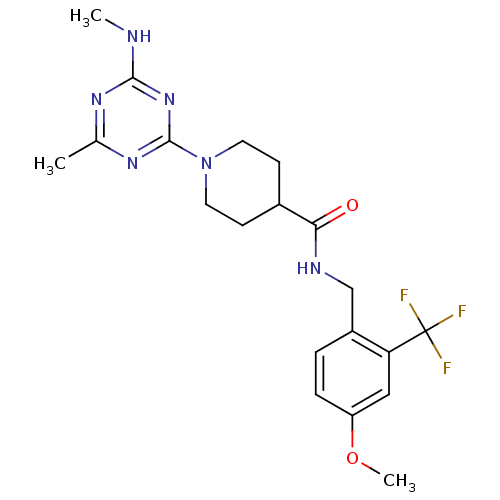
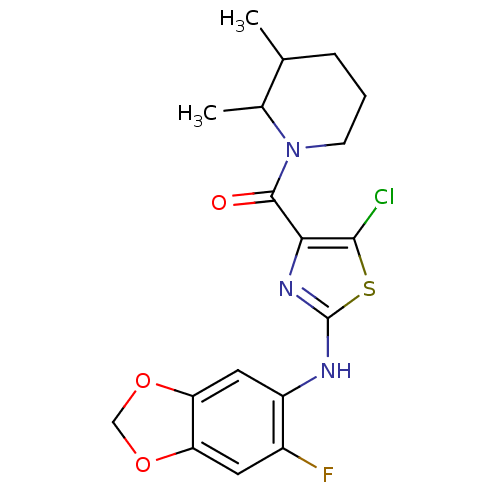
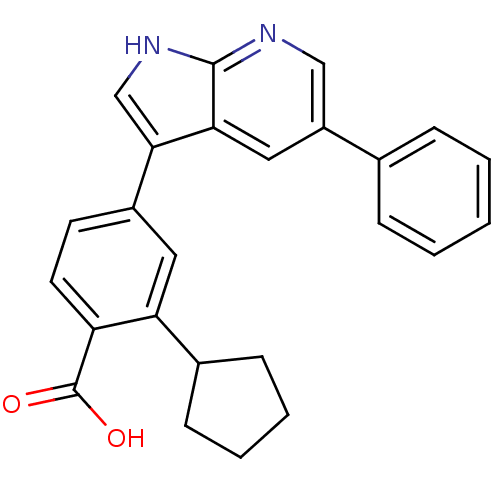
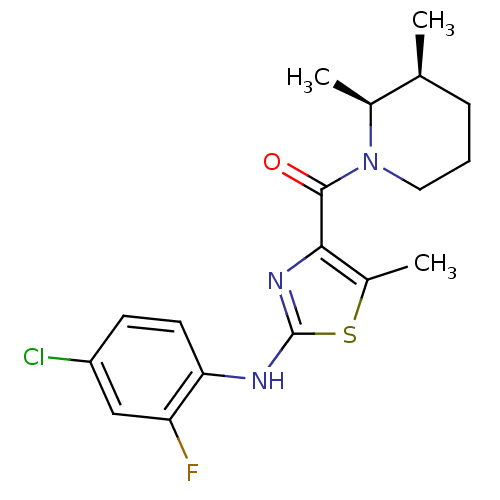
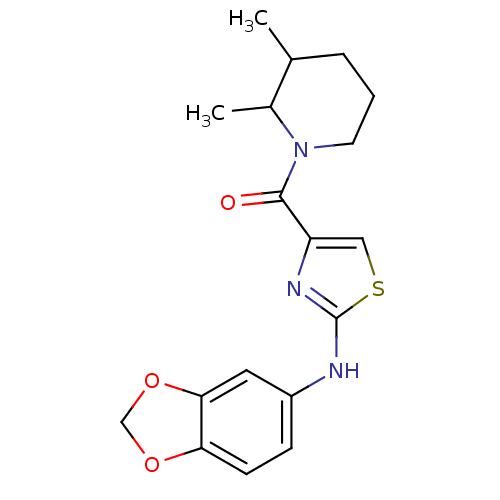
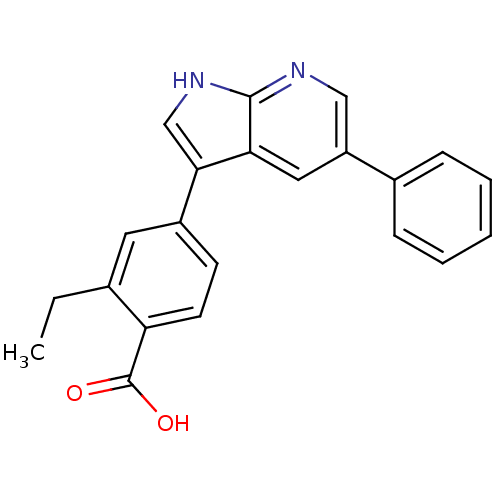
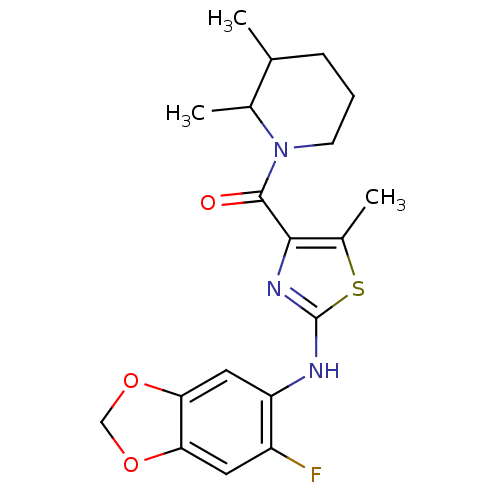
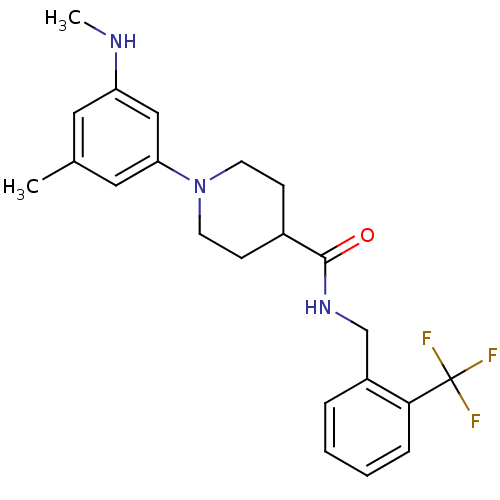
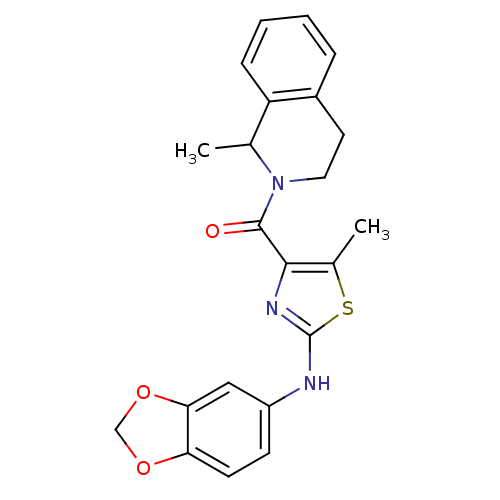
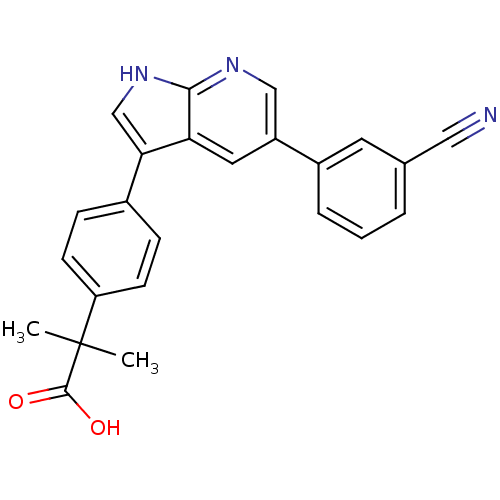
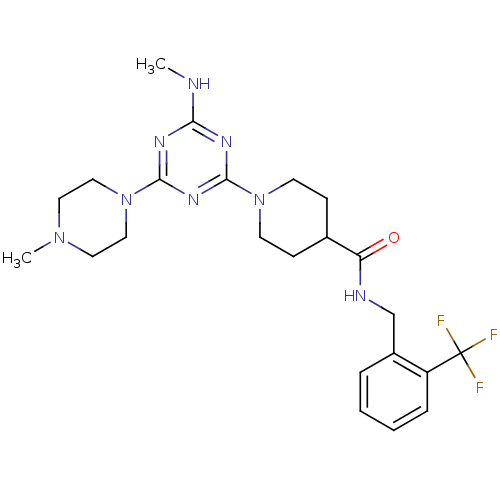
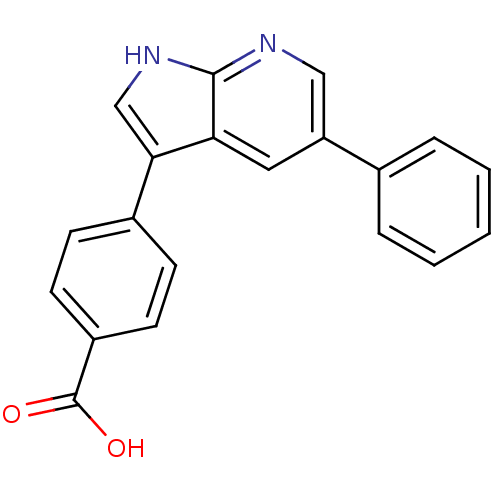
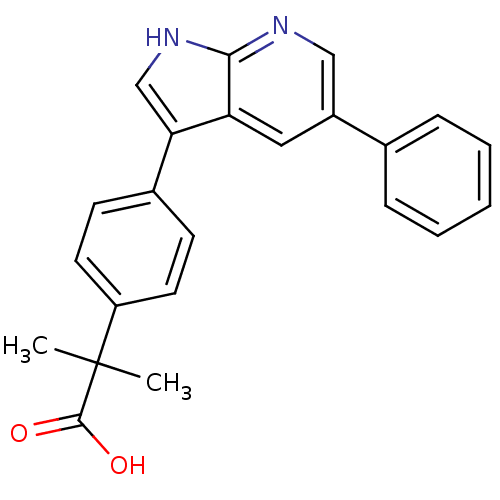
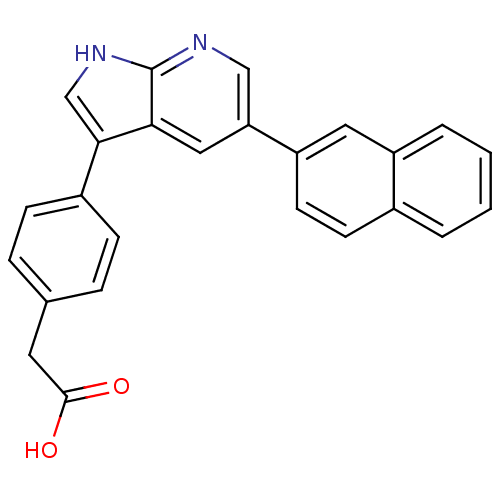
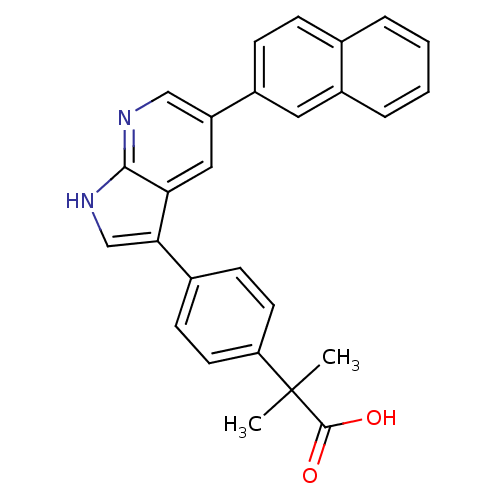
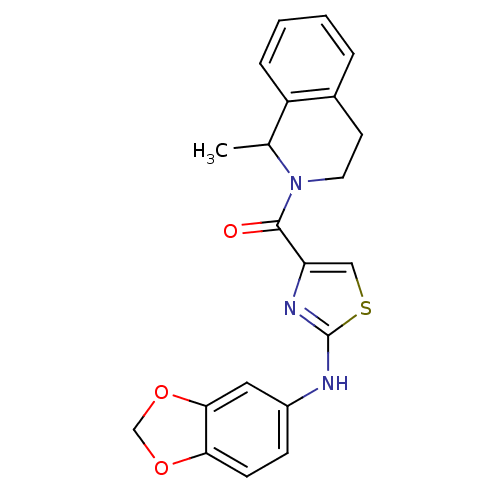
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
TargetShort transient receptor potential channel 3(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of human recombinant TRPC3 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2WQ03VCPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2WQ03VCPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 40nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2WQ03VCPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2WQ03VCPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
TargetShort transient receptor potential channel 6(Homo sapiens (Human))
GlaxoSmithKline
Curated by ChEMBL
GlaxoSmithKline
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of human recombinant TRPC6 expressed in HEK293-MSRII cells assessed as carbachol-stimulated Ca2+/Na+ influx after 10 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio...More data for this Ligand-Target Pair