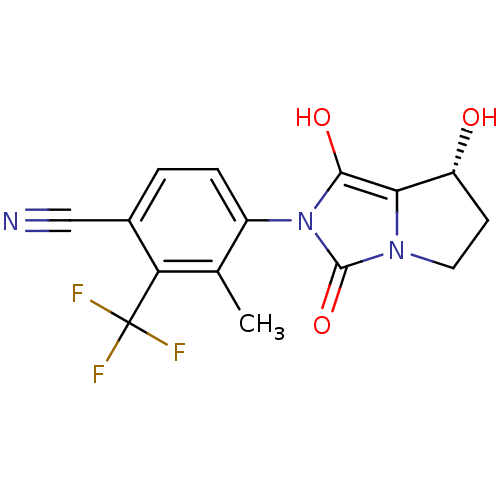
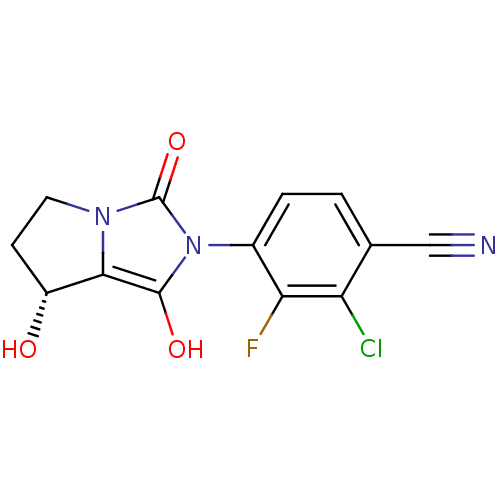
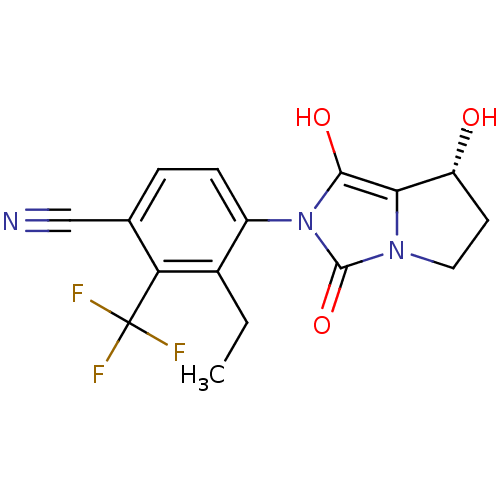
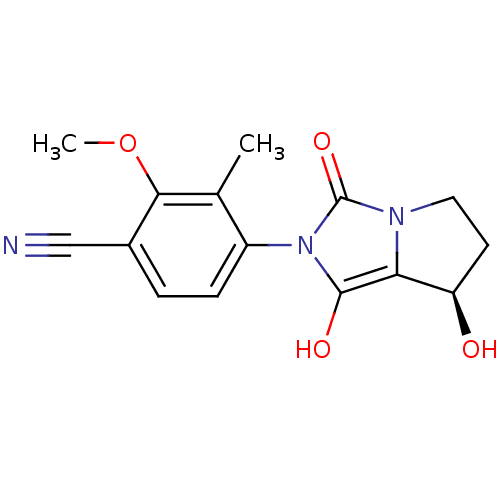
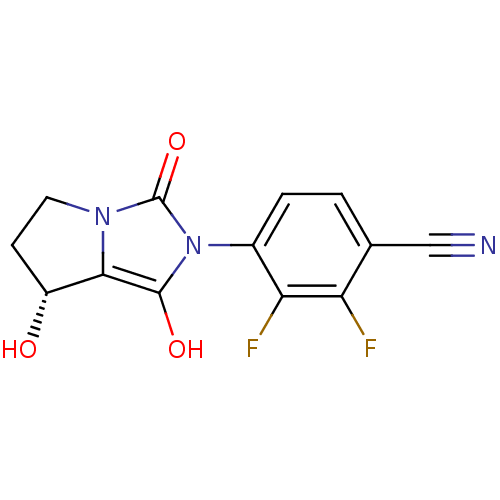
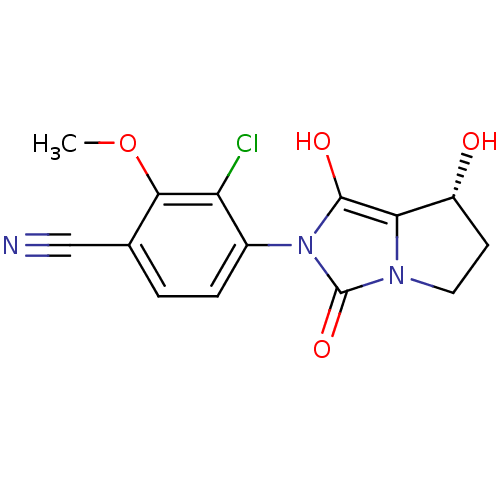
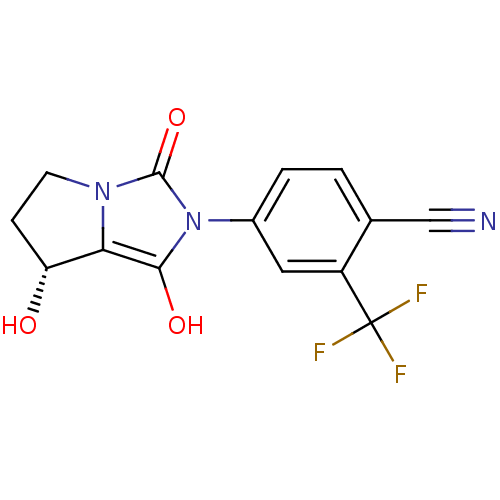
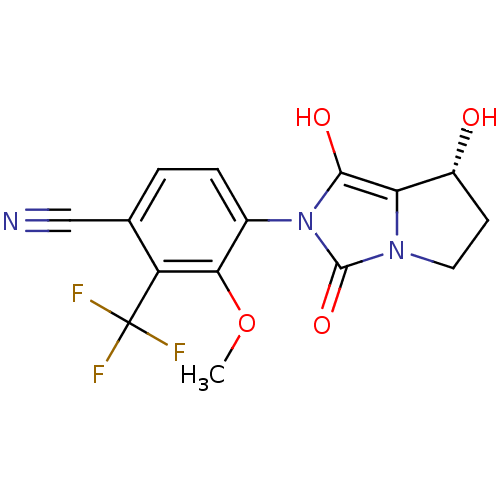
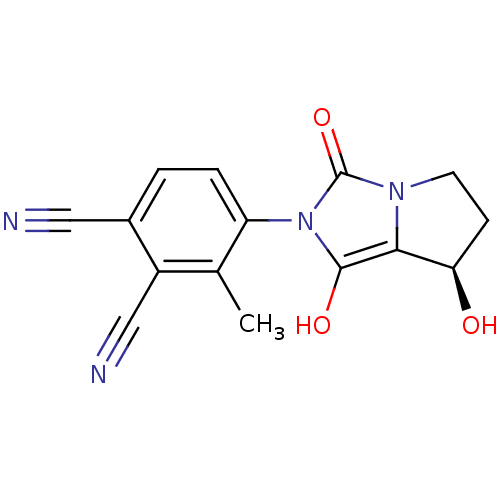
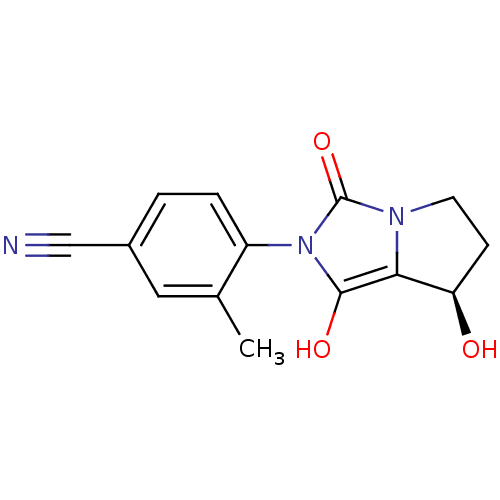
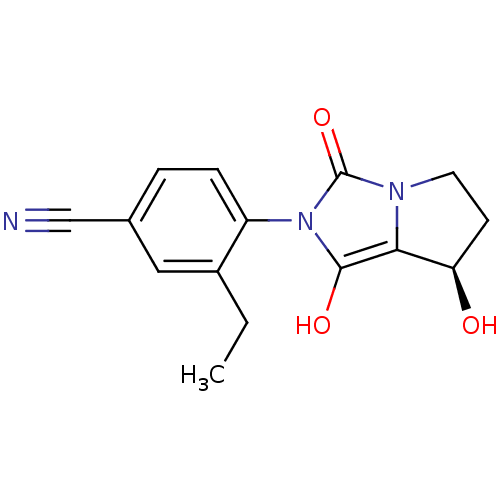
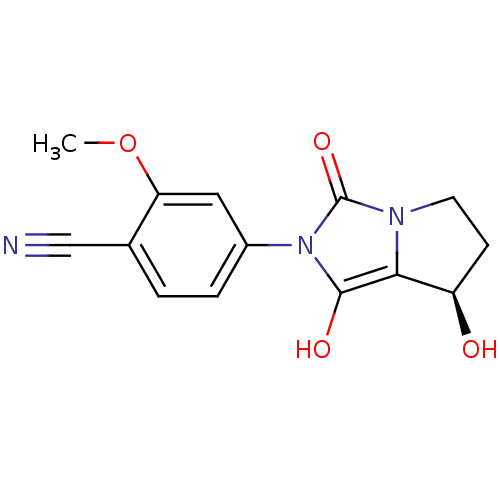
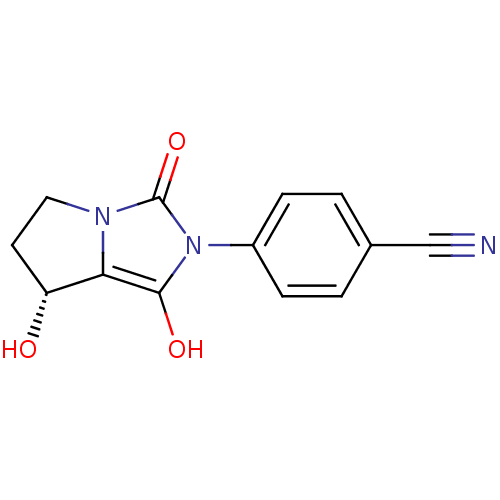
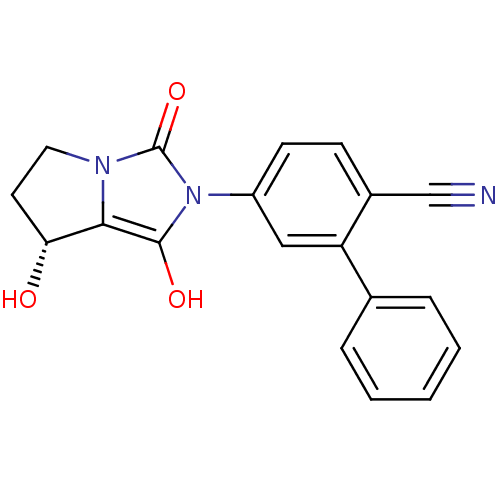
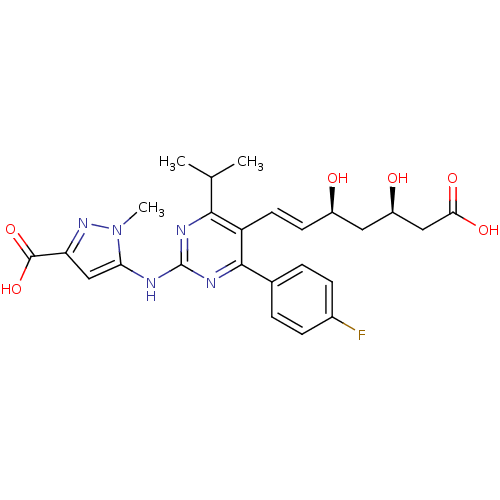
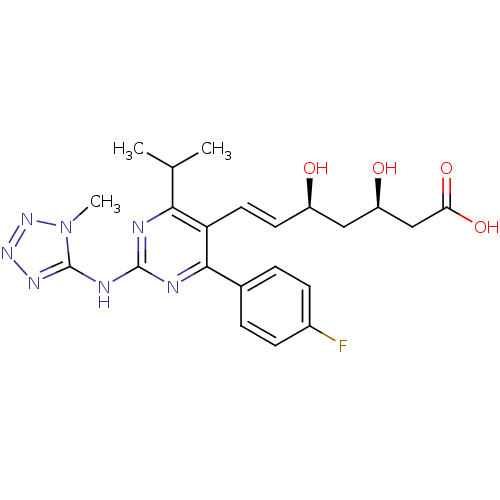
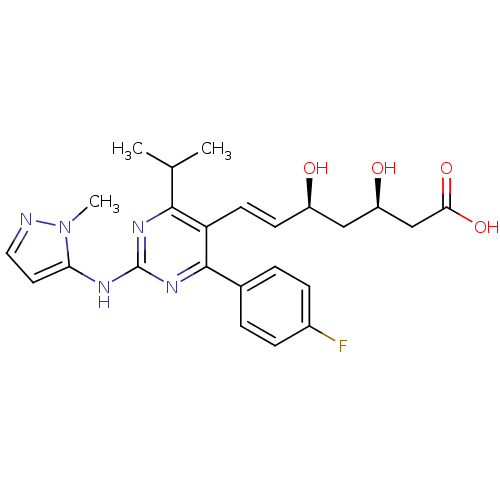
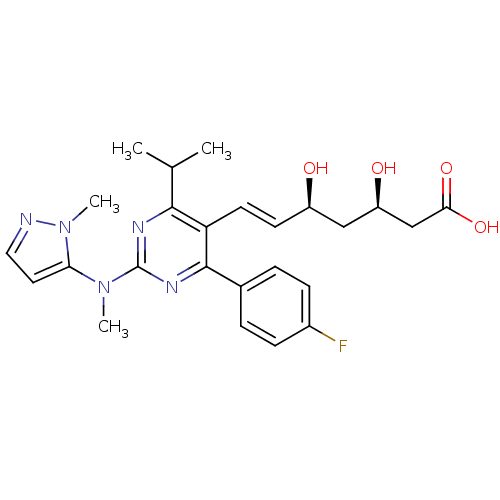
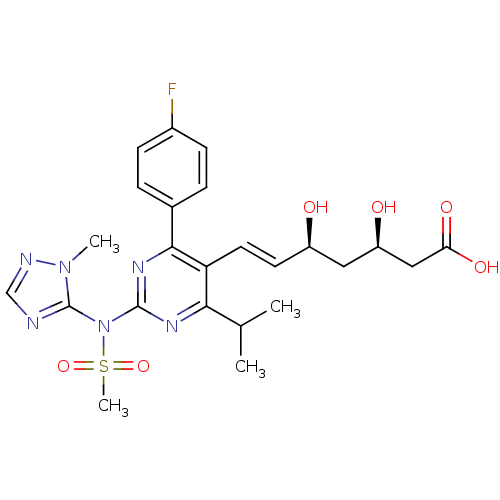
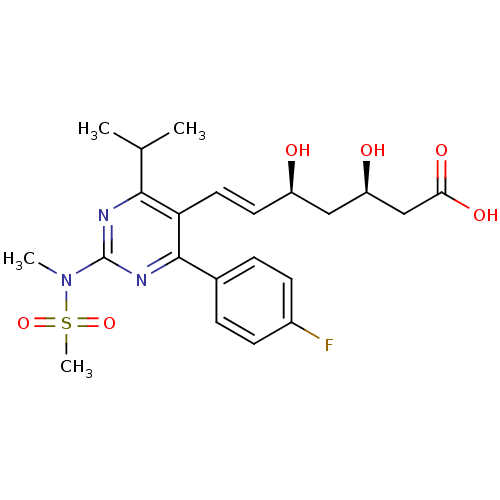
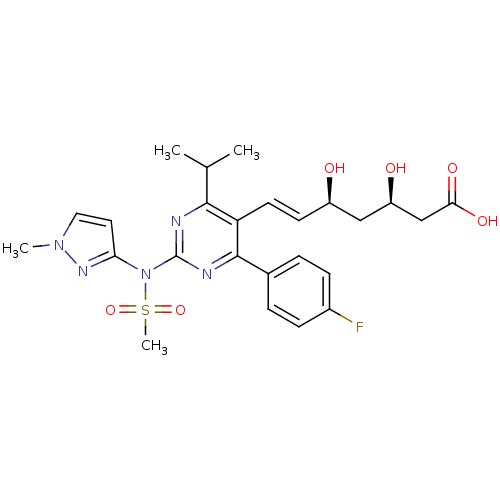
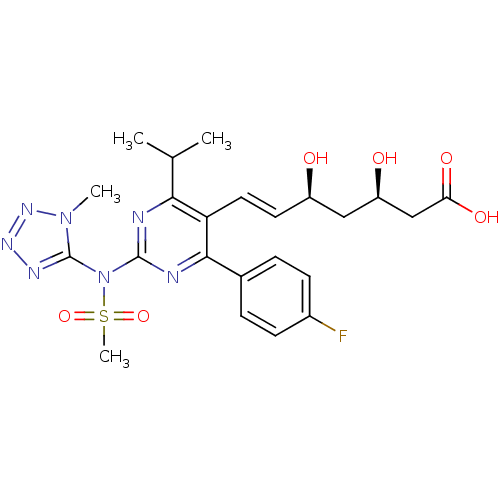
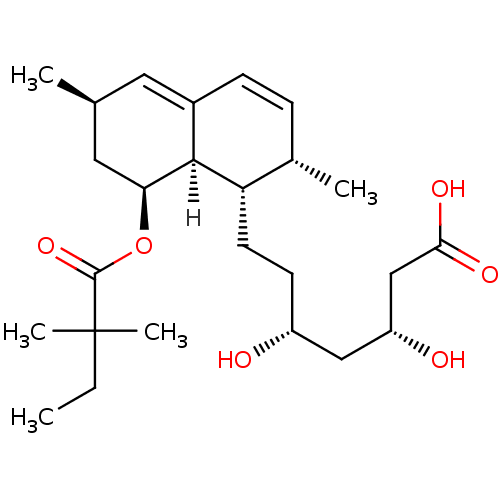
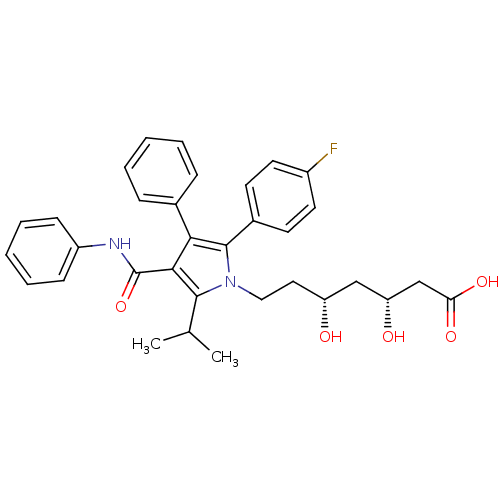
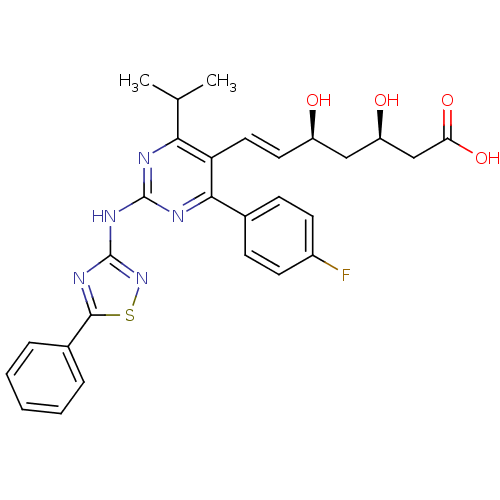
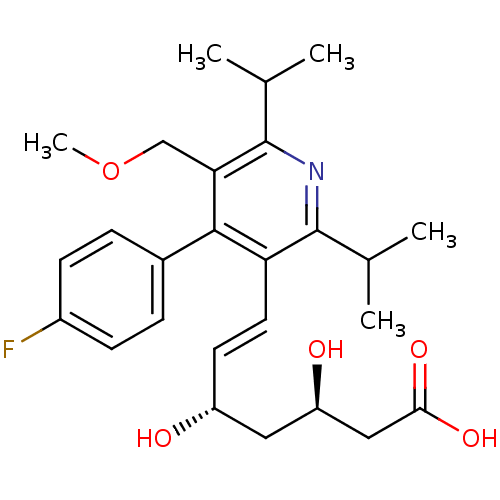
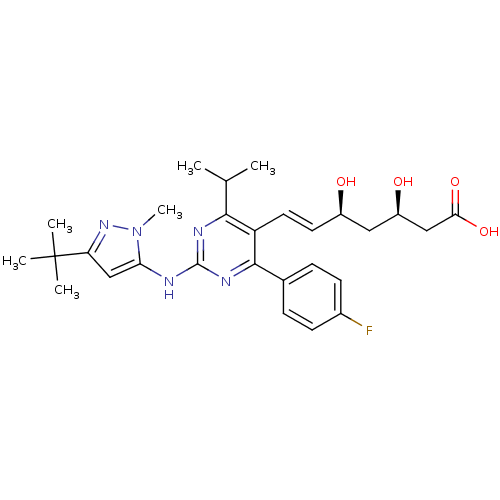
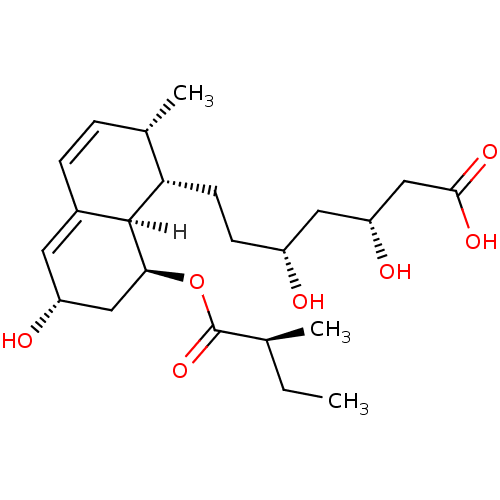
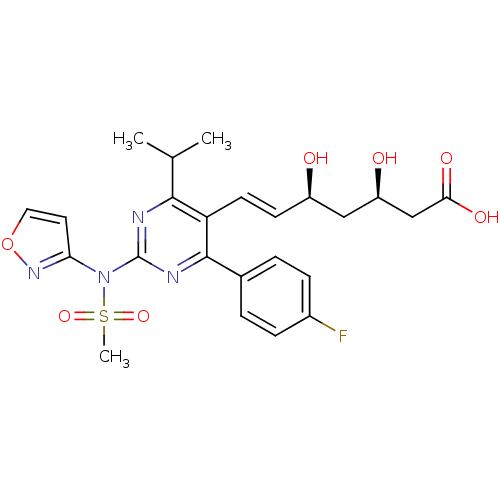
Affinity DataKi: 0.700nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 7.80nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 7.90nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 8.10nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 8.5nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Binding affinity to human ARMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 0.5nM EC50: 1.40nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 0.900nM EC50: 13.7nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 0.900nM EC50: 1.5nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 1.40nM EC50: 3.95nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 1.80nM EC50: 3.70nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 2.20nM EC50: 5.40nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 2.30nM EC50: 1.40nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 2.40nM EC50: 1.30nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 2.5nM EC50: 2.5nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 2.5nM EC50: 1.20nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 2.90nM EC50: 7.5nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 3nM EC50: 3.70nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 3.10nM EC50: 0.600nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 3.70nM EC50: 7.70nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 4.20nM EC50: 1nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 4.30nM EC50: 6.20nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 6.20nM EC50: 2.5nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 6.90nM EC50: 7.70nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 7nM EC50: 9nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 9.80nM EC50: 2.30nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 12.4nM EC50: 1.90nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 31.6nM EC50: 29nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Rattus norvegicus (rat))
Bristol-Myers Squibb Company
Bristol-Myers Squibb Company
Affinity DataIC50: 33nM EC50: 5.5nMpH: 7.0 T: 2°CAssay Description:Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme...More data for this Ligand-Target Pair
Affinity DataEC50: 79nMAssay Description:Agonist activity at human ARMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human ARMore data for this Ligand-Target Pair
Affinity DataEC50: 2.30nMAssay Description:Agonist activity at human ARMore data for this Ligand-Target Pair
Affinity DataEC50: 47nMAssay Description:Agonist activity at human ARMore data for this Ligand-Target Pair

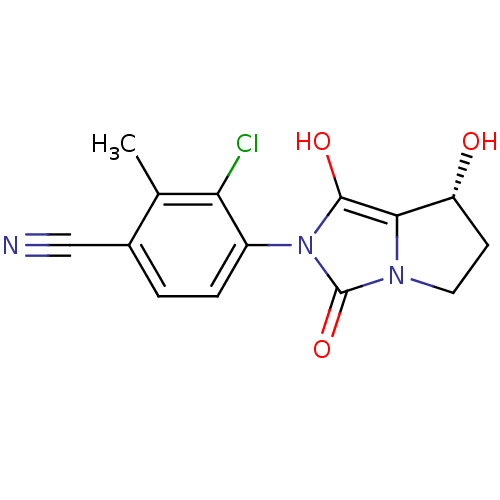
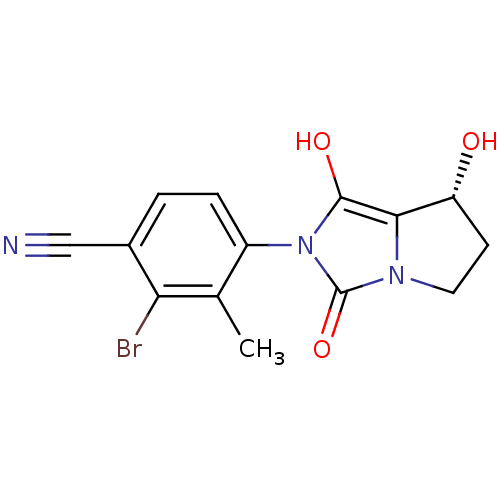
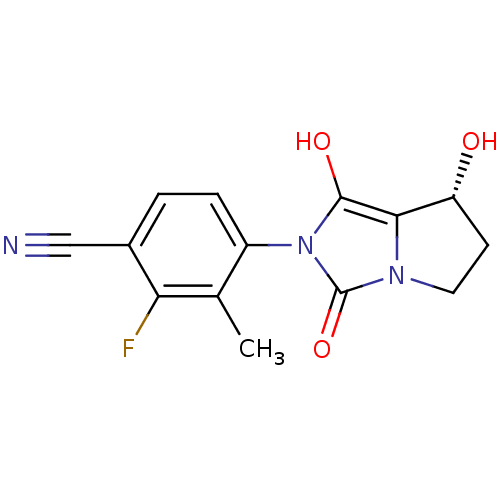
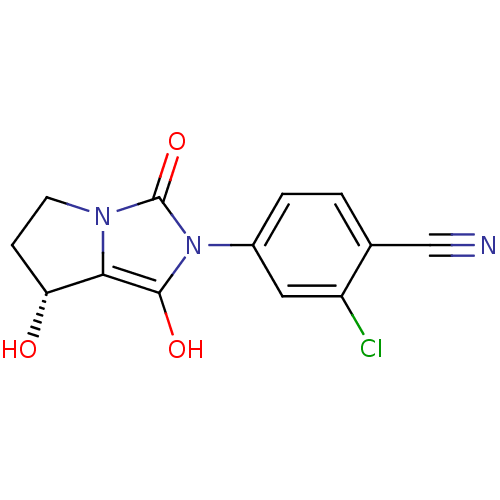
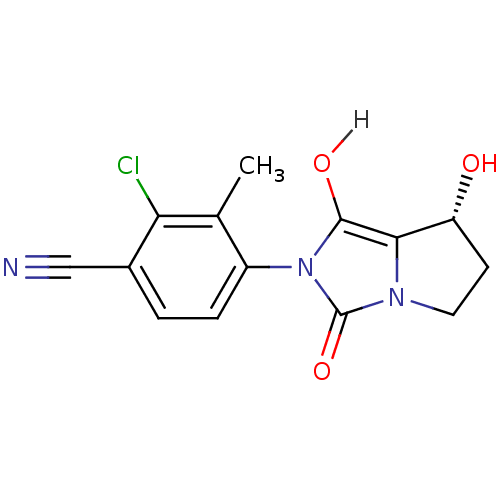
 3D Structure (crystal)
3D Structure (crystal)