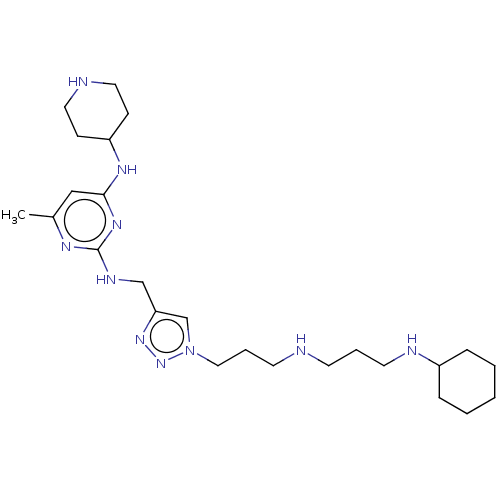
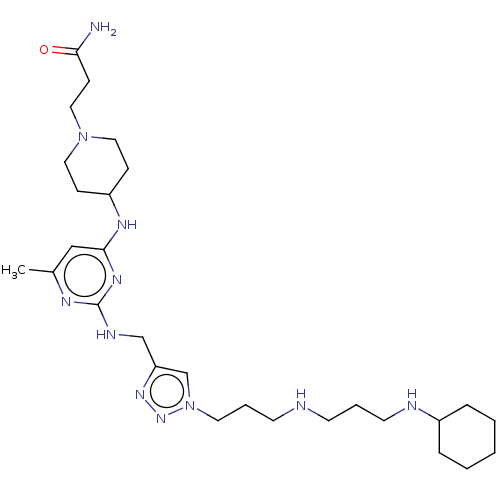
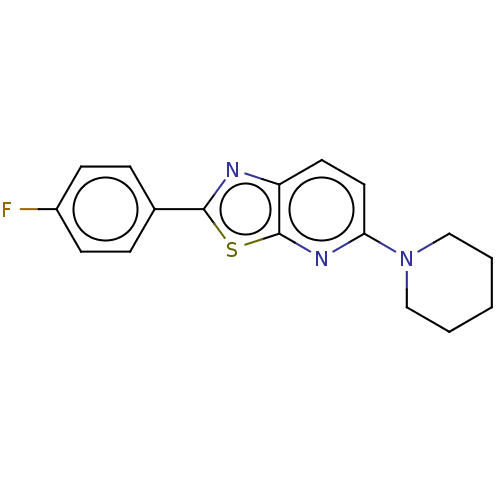
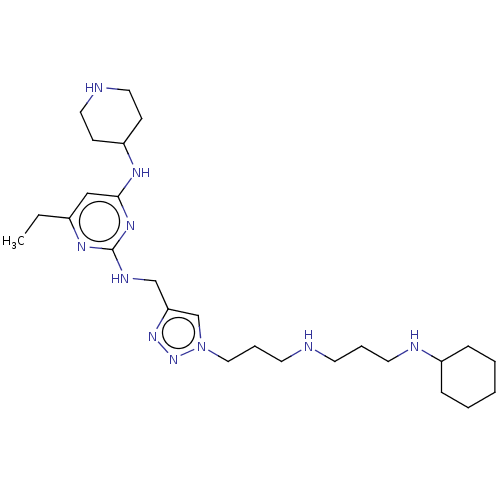
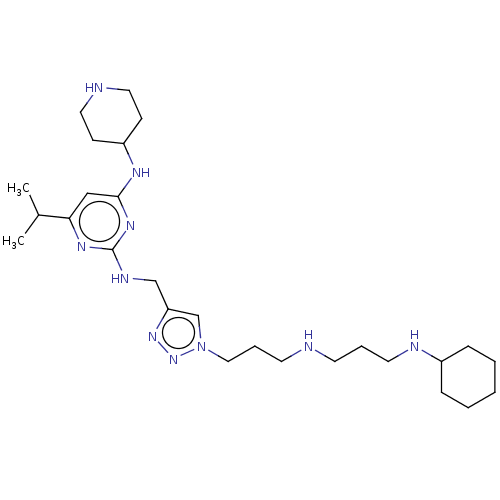
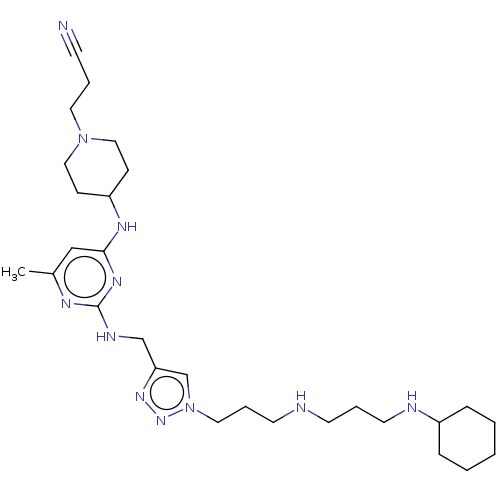
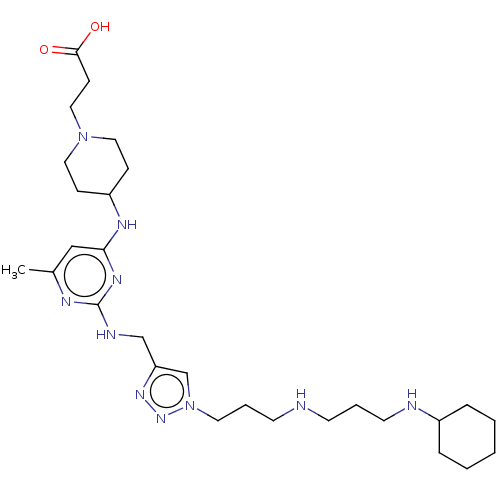
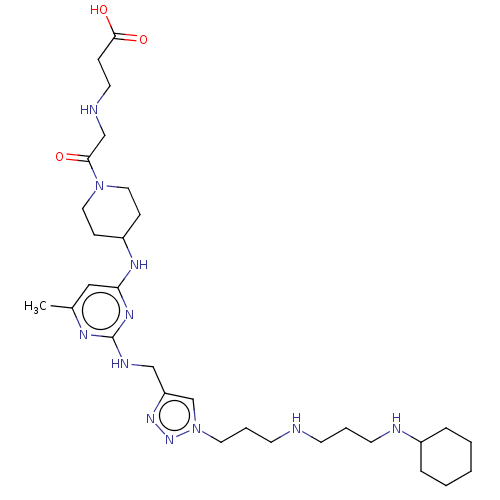
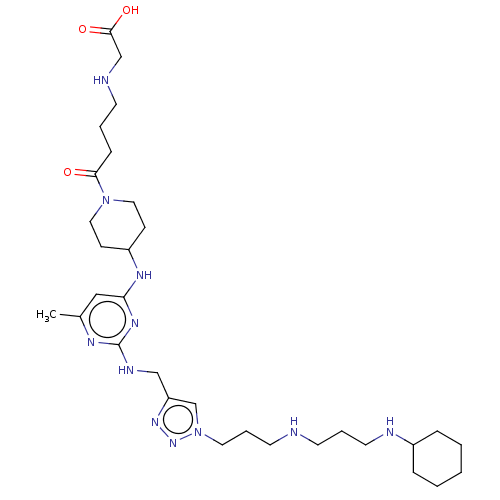
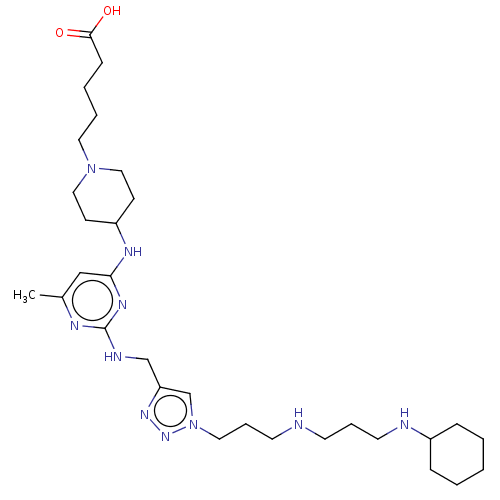
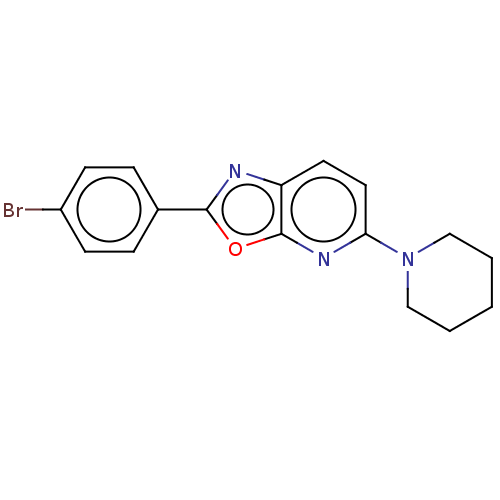
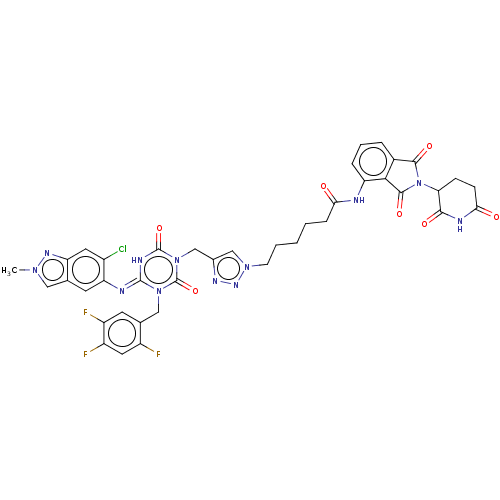
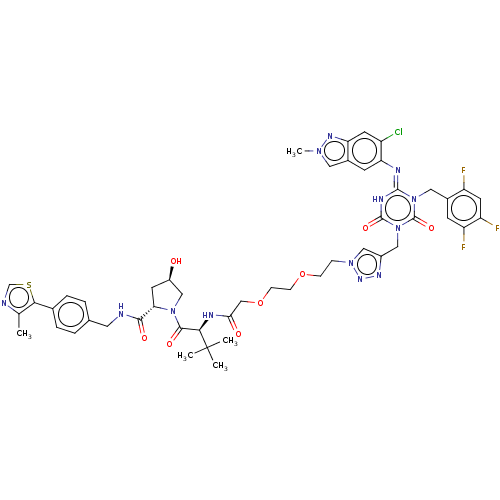
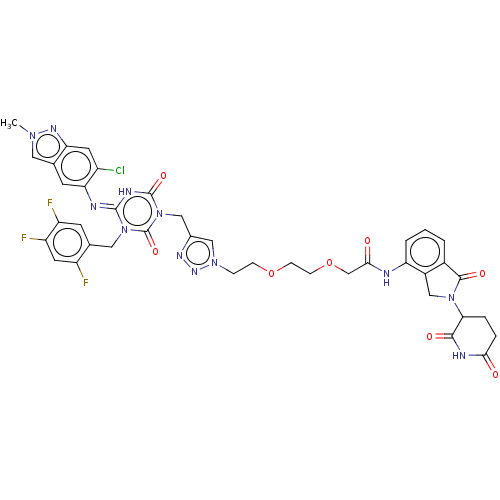
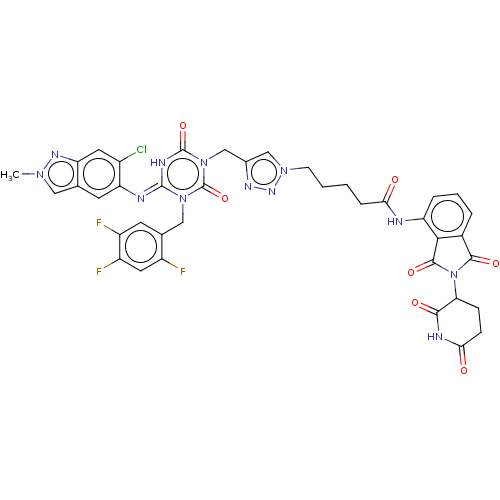
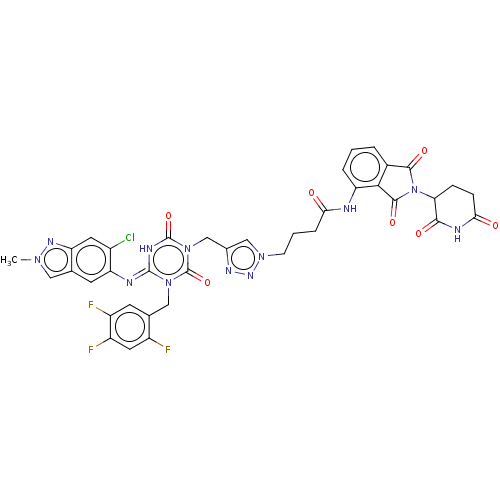
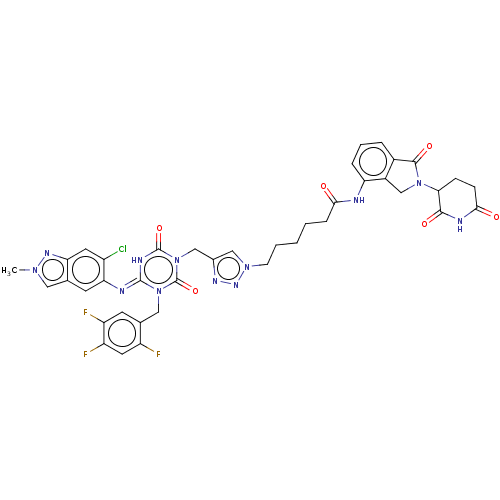
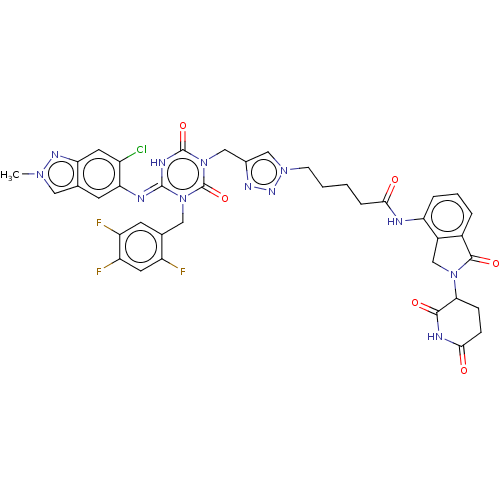
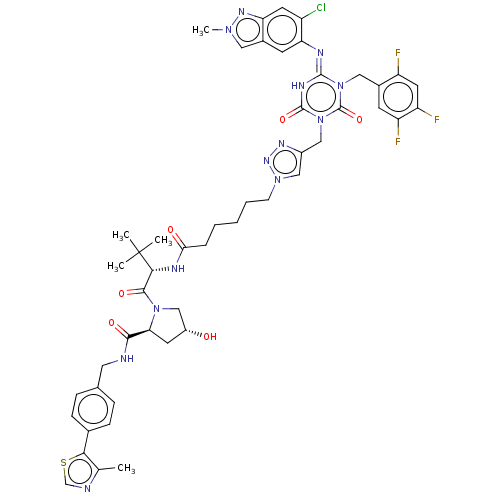
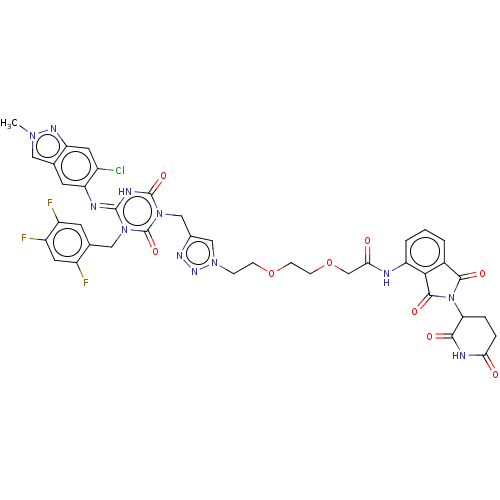
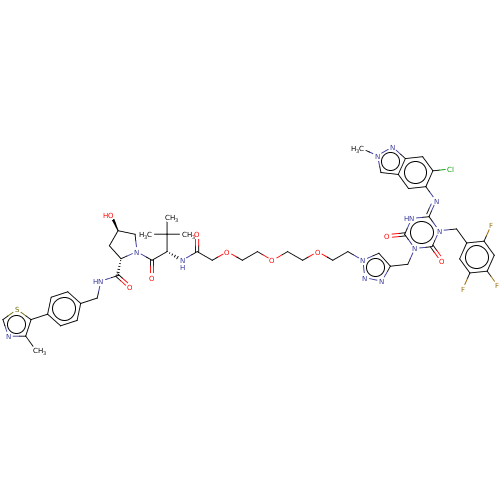
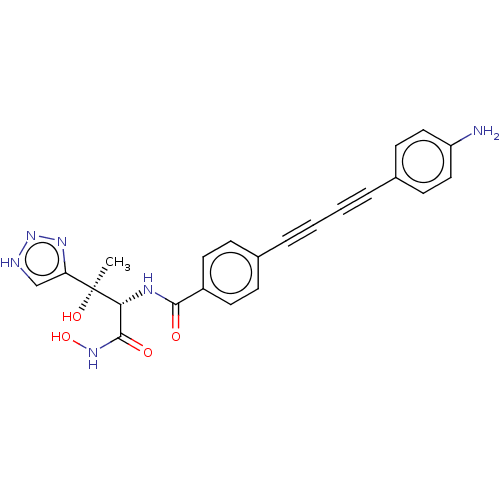
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.0240nMAssay Description:Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy...More data for this Ligand-Target Pair
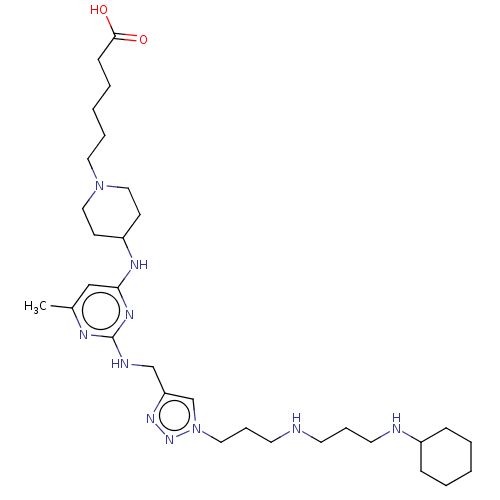
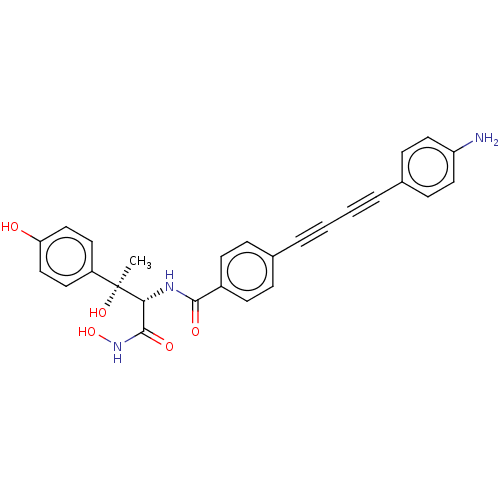
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.0240nMAssay Description:Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy...More data for this Ligand-Target Pair
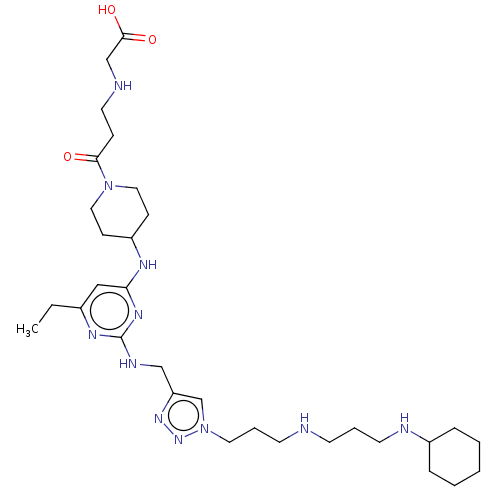
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of Escherichia coli LpxCMore data for this Ligand-Target Pair
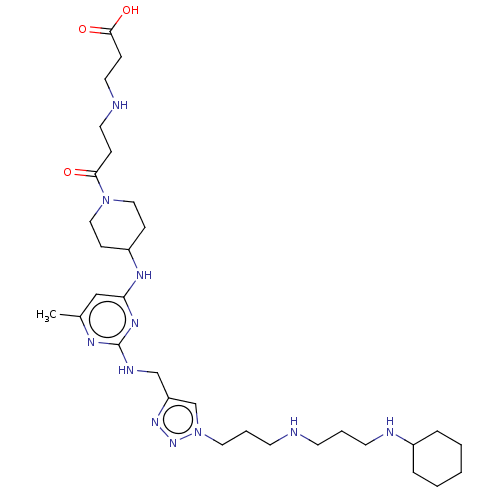
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of Escherichia coli LpxCMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of Escherichia coli LpxC enzymeMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.550nMAssay Description:Inhibition of Escherichia coli LpxCMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 0.550nMAssay Description:Inhibition of Escherichia coli LpxCMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Duke University
Curated by ChEMBL
Duke University
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Inhibition of Escherichia coli LpxC enzymeMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 61nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 71nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 83nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 89nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 92nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 93nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 96nMAssay Description:A stock solution was prepared using a human MAO-B enzyme (purchased from Aldrich) and a Amplex Red monoamine oxidase assay kit according to a prepara...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 98nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The inhibitory activity of the compounds against SARS-CoV-2 3CLpro was determined using fluorescence resonance energy transfer.10 μL of the comp...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 147nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 158nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 171nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 213nMAssay Description:Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 267nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:A stock solution was prepared using a human MAO-B enzyme (purchased from Aldrich) and a Amplex Red monoamine oxidase assay kit according to a prepara...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 272nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Korea Institute of Science and Technology
Curated by ChEMBL
Korea Institute of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 335nMAssay Description:Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assayMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)