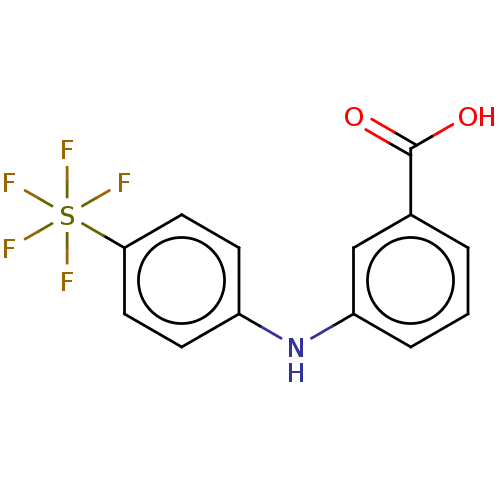
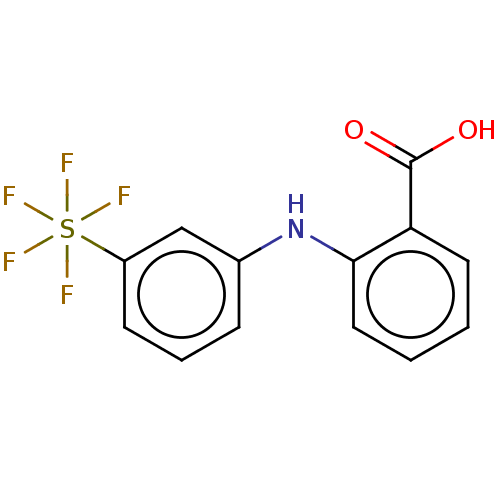
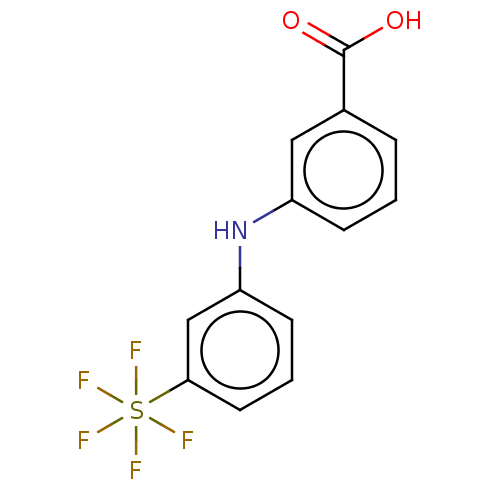
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of recombinant AKR1C3 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
RWTH Aachen University
Curated by ChEMBL
RWTH Aachen University
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant AKR1C2 (unknown origin) using S-tetralol as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX1 (unknown origin) using arachidonic acid substrate by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 2.90E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 2.80E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+6nMAssay Description:Binding affinity to rat bile acid-sensitive ion channel expressed in xenopus oocytesMore data for this Ligand-Target Pair