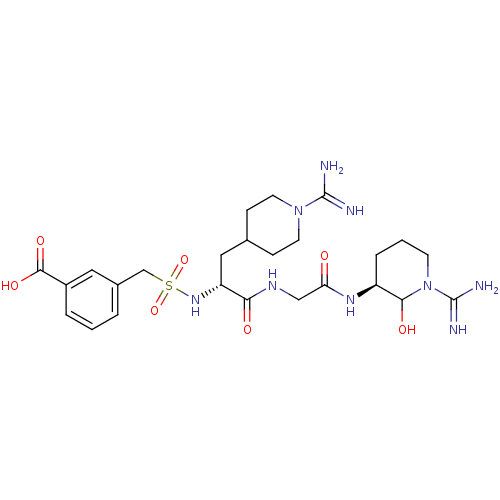
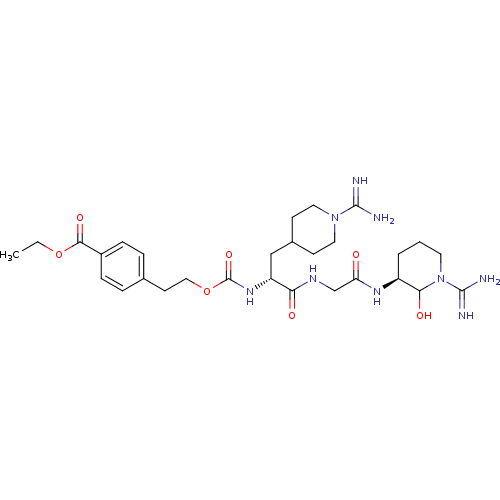
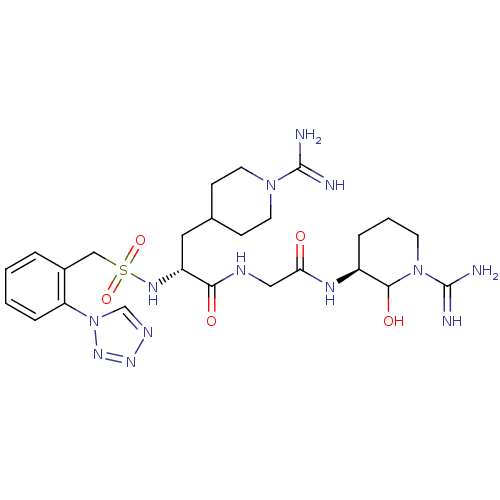
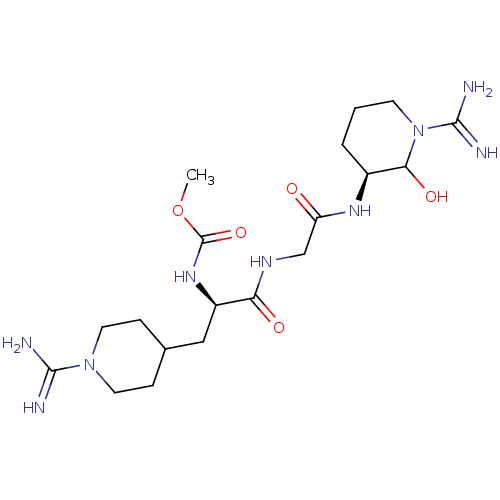
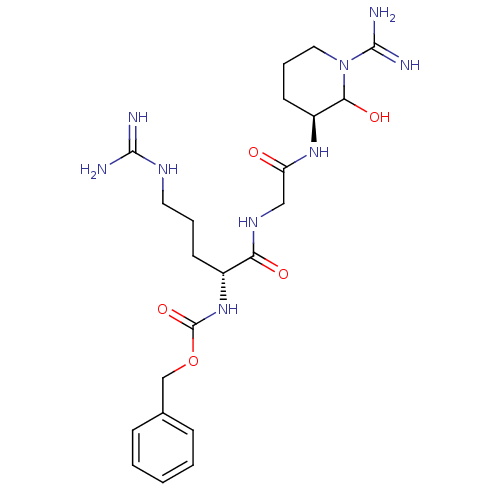
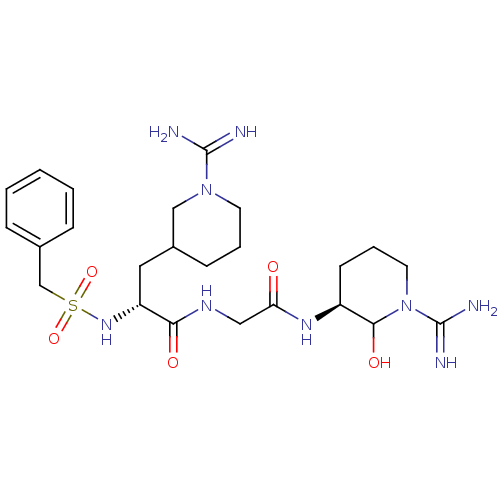
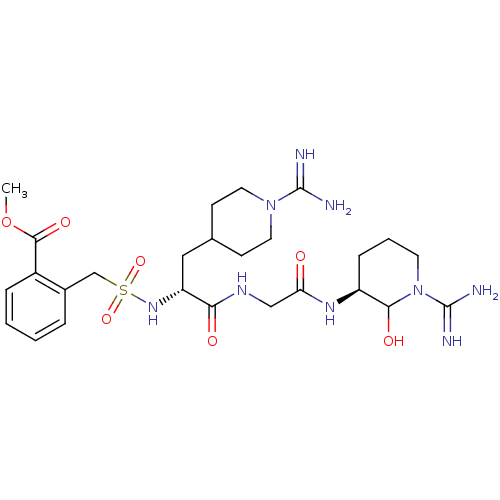
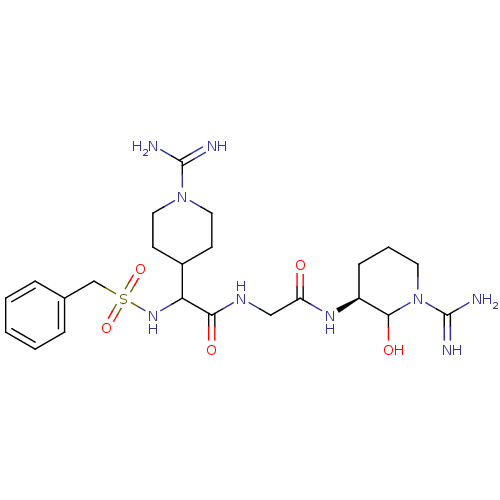
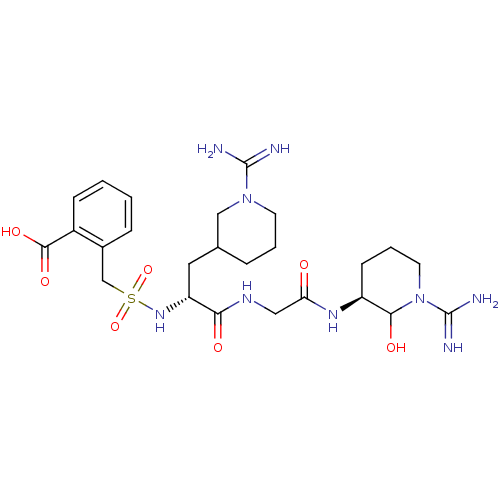
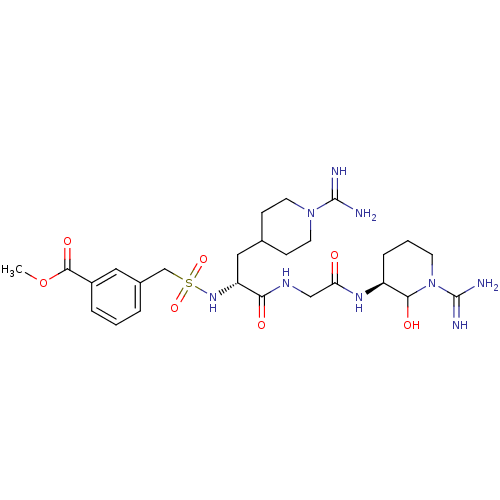
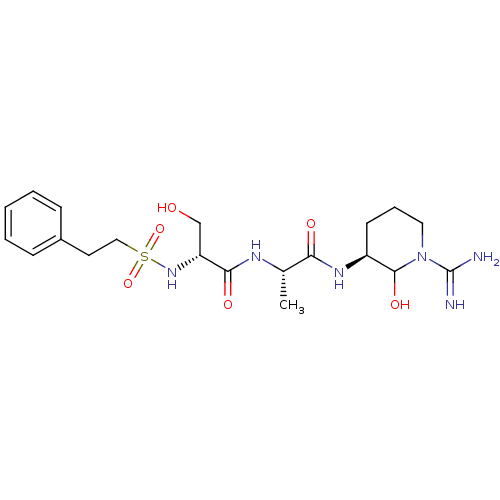
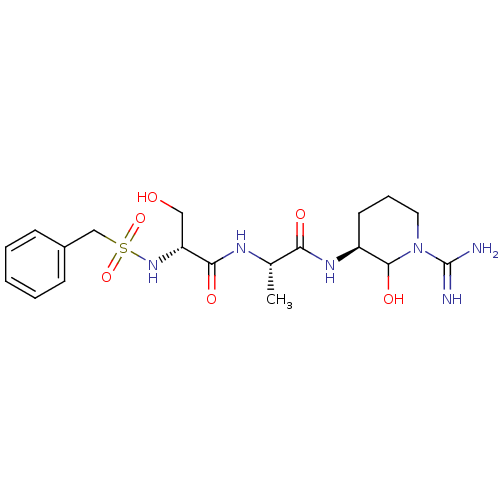
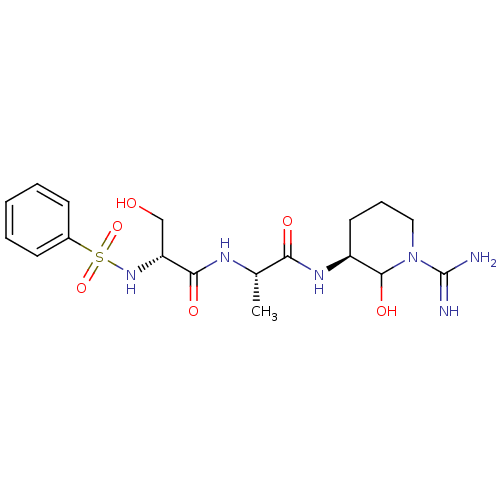
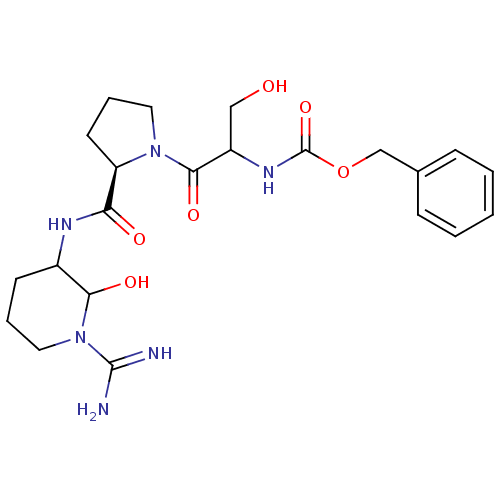
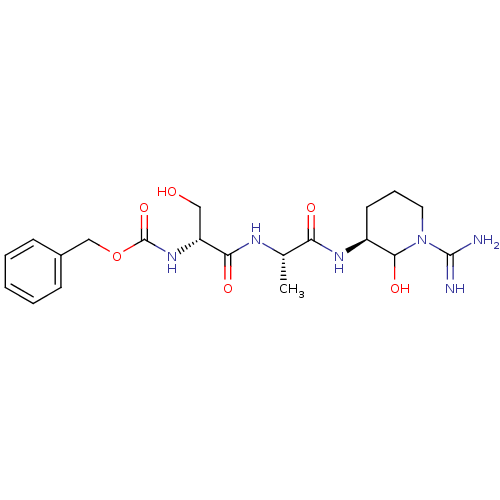
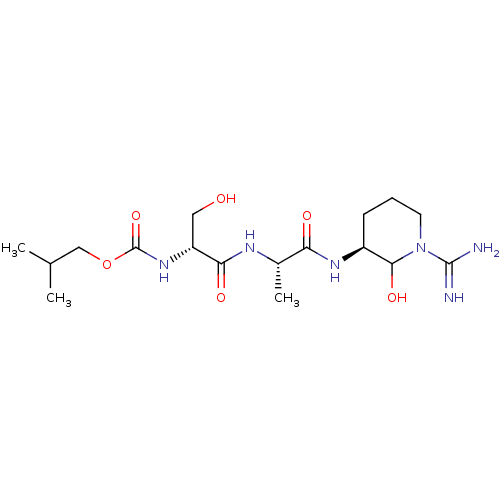
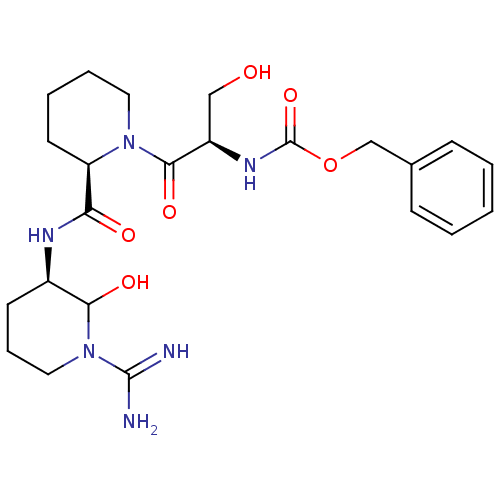
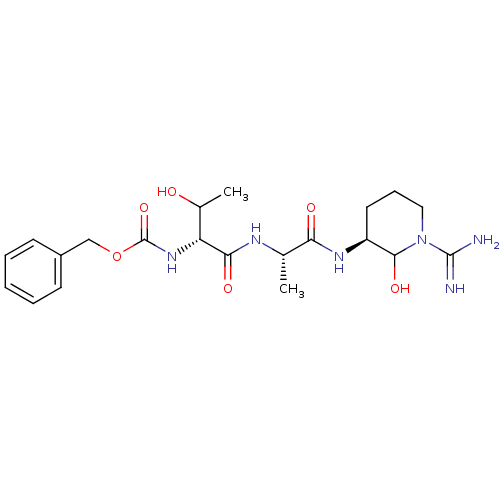
Affinity DataKi: 16nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:In vitro inhibitory activity against human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Concentration of the compound required for classical fast inhibition of cleavage of the chromogenic substrate by human enzyme Coagulation factor X in...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:In vitro inhibitory activity against human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 0.830nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 1.37nMAssay Description:Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISAMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Concentration of the compound required to inhibit the cleavage of the chromogenic substrate by human enzyme Coagulation factor X in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:In vitro inhibition of human plasmin.More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 101nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 115nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 145nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 147nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 158nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 169nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 211nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 231nMAssay Description:In vitro inhibition of human plasmin.More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Corvas International, Inc.
Curated by ChEMBL
Corvas International, Inc.
Curated by ChEMBL
Affinity DataIC50: 261nMAssay Description:The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 275nMAssay Description:The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 352nMAssay Description:In vitro inhibition of human thrombin.More data for this Ligand-Target Pair
Affinity DataIC50: 367nMAssay Description:The compound was tested in vitro for its inhibitory activity against human Plasmin enzyme, activity expressed as IC50More data for this Ligand-Target Pair