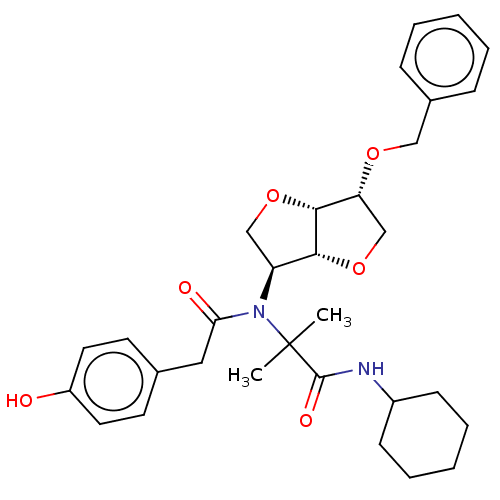
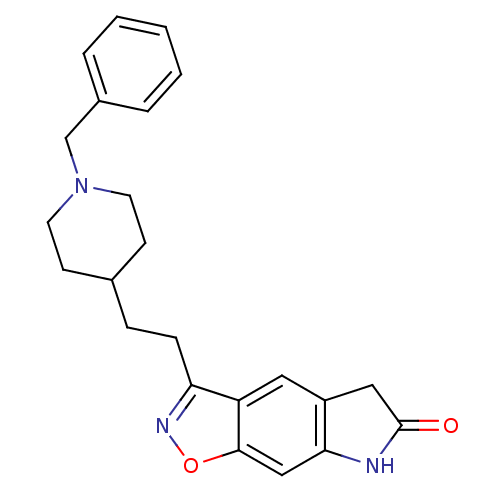
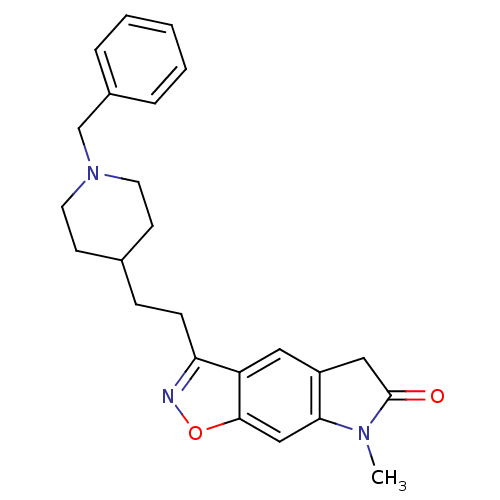
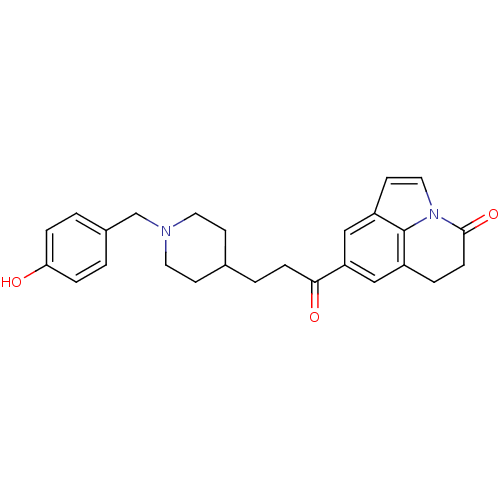
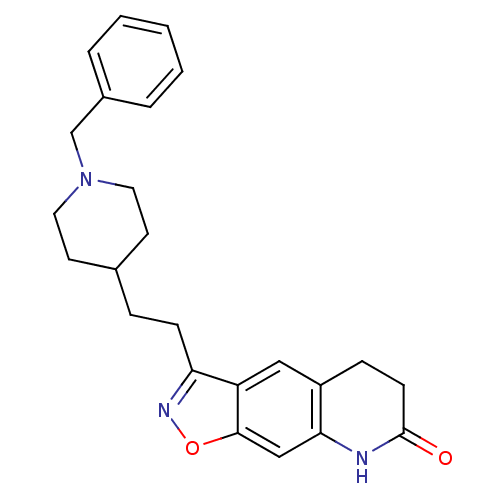
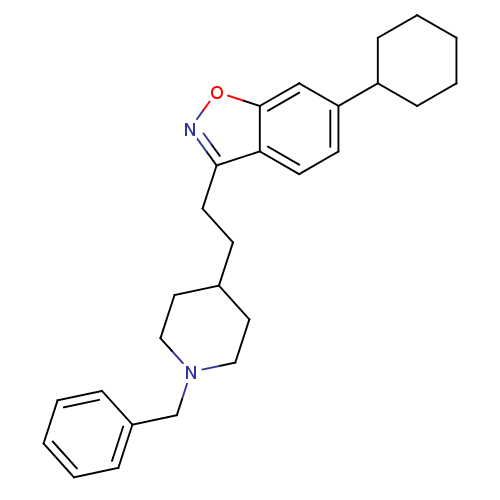
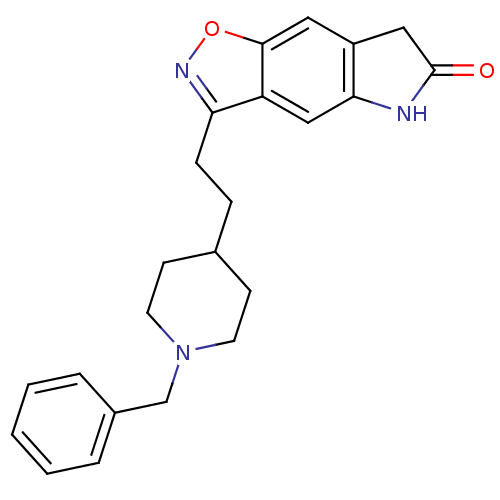
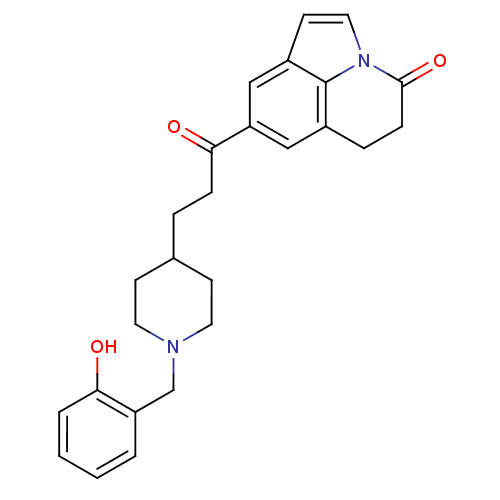
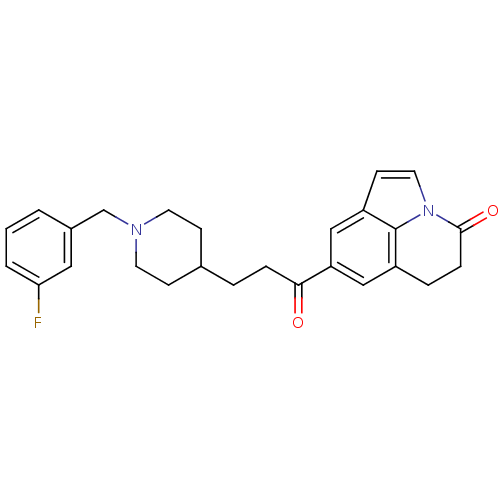
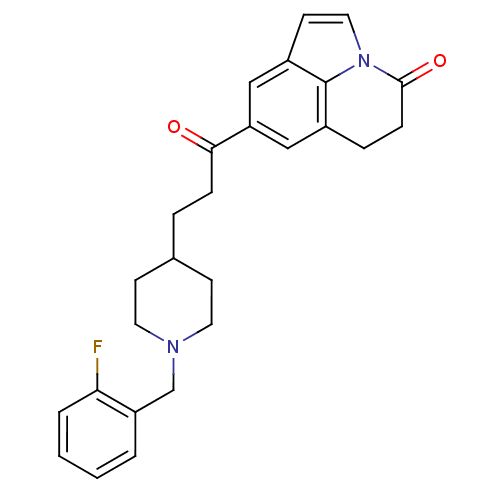
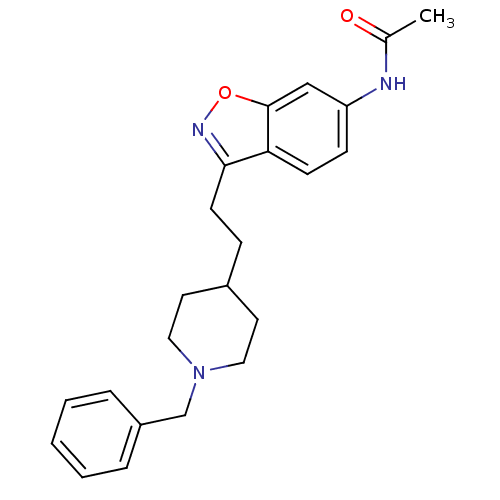
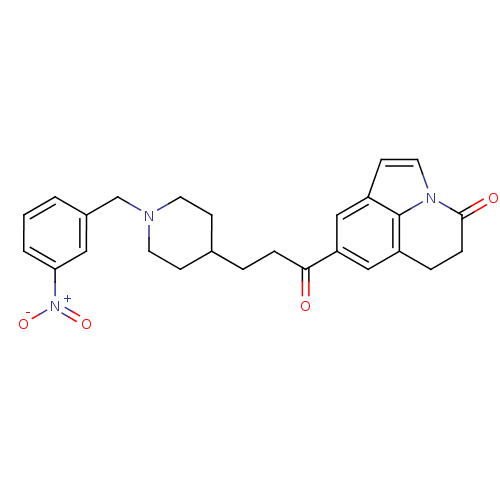
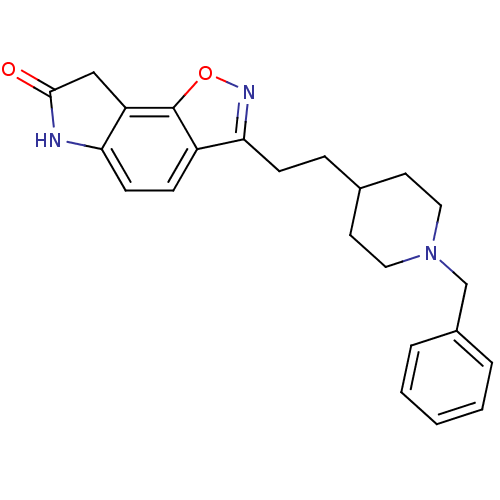
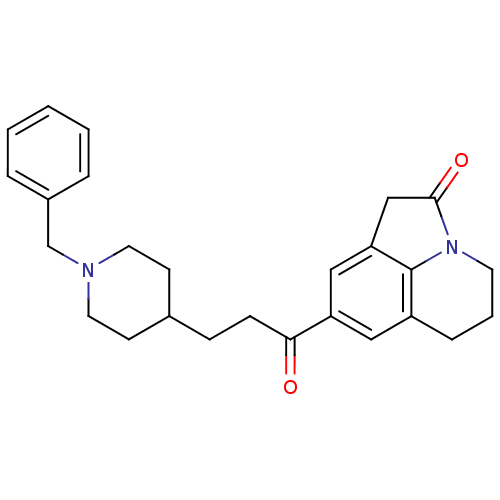
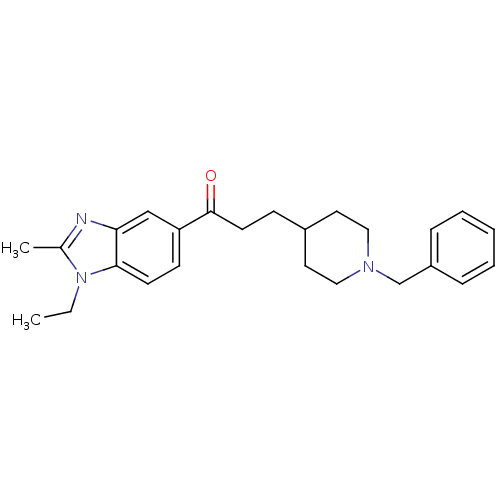
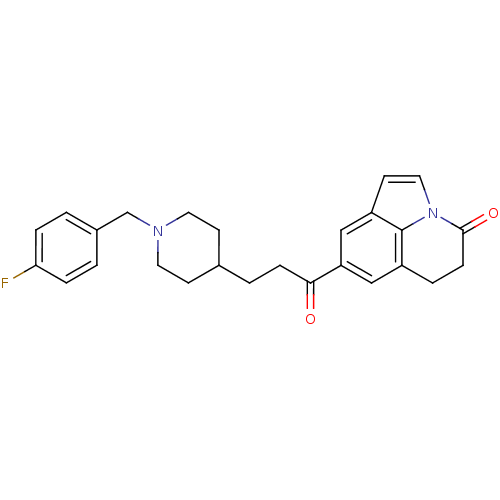
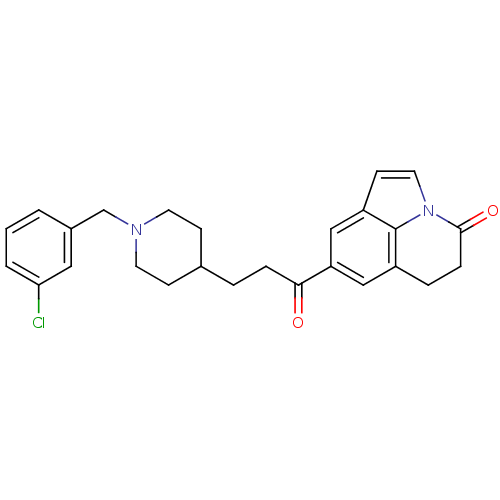
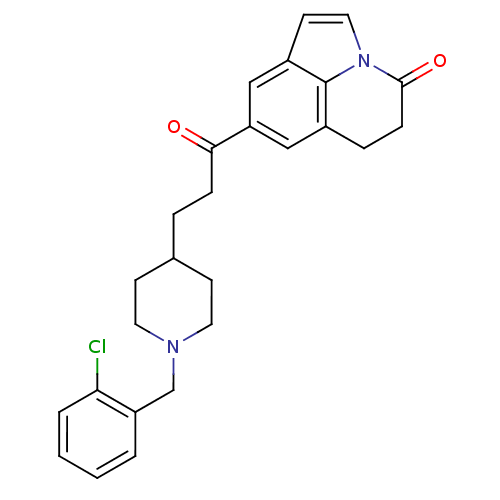
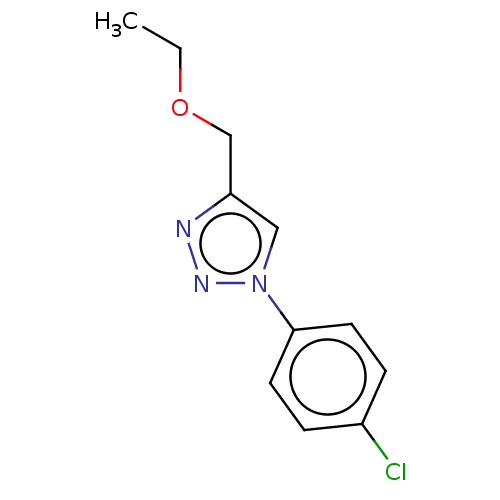
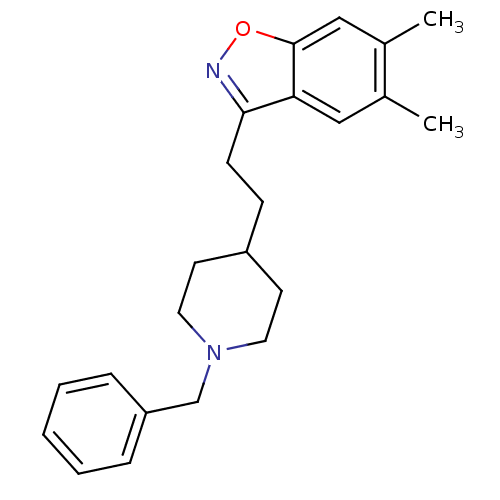
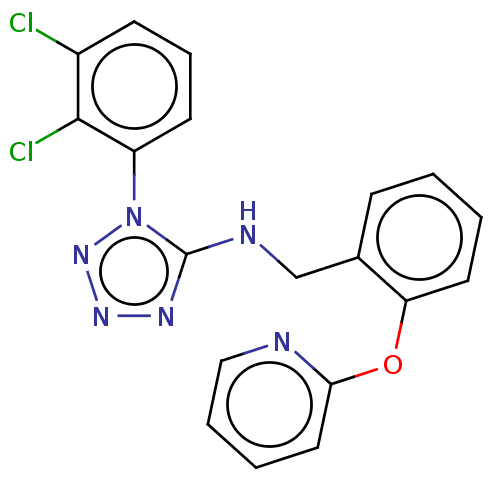
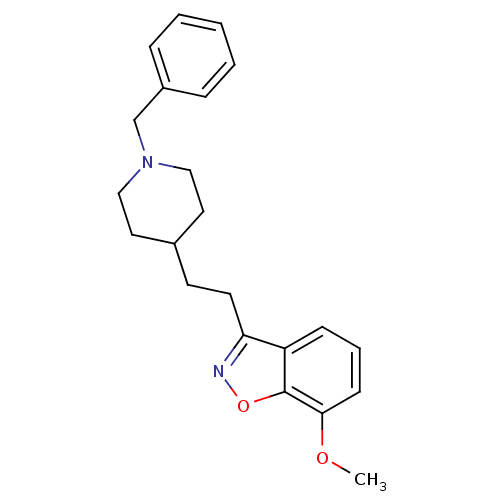
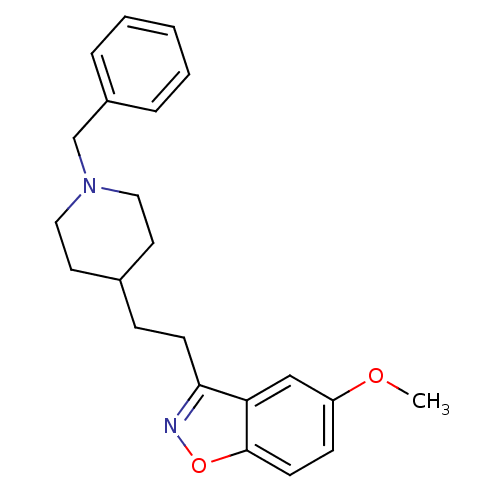
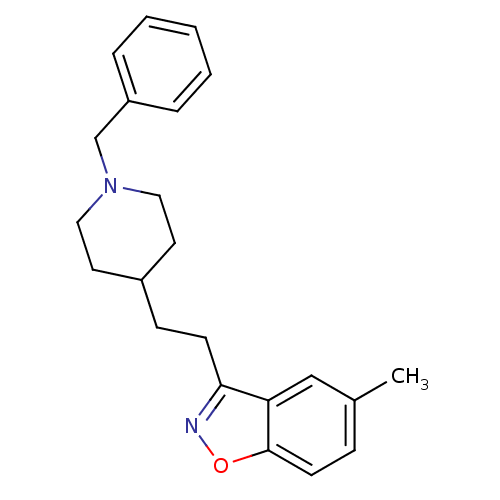
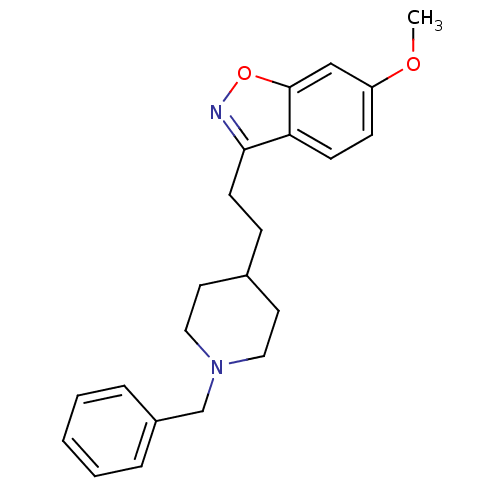
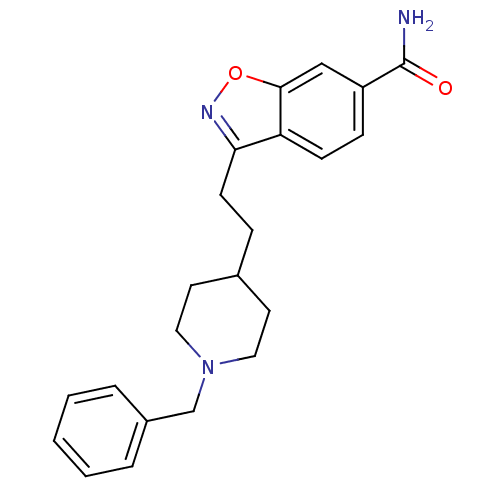
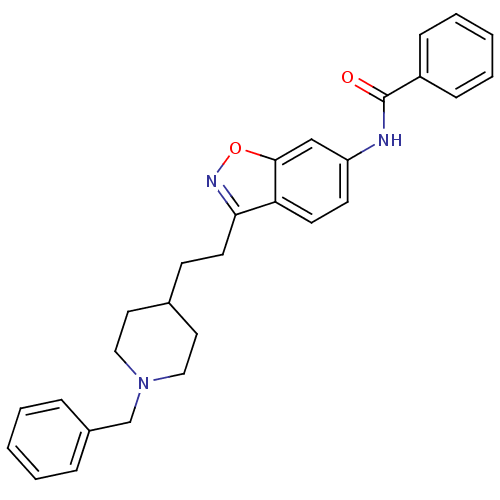
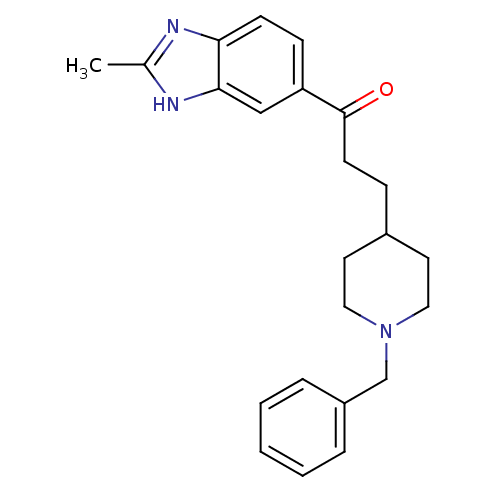
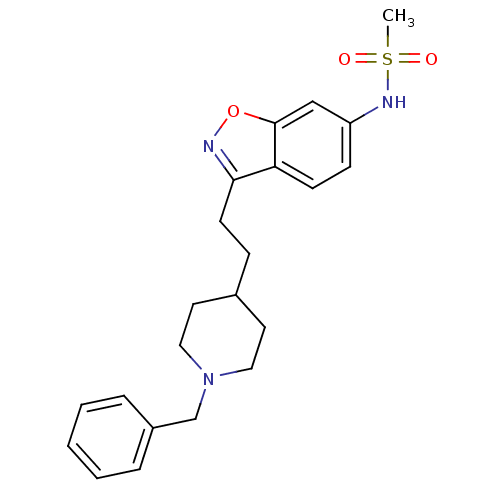
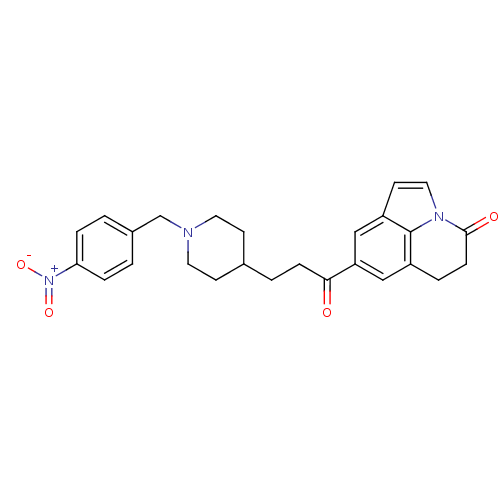
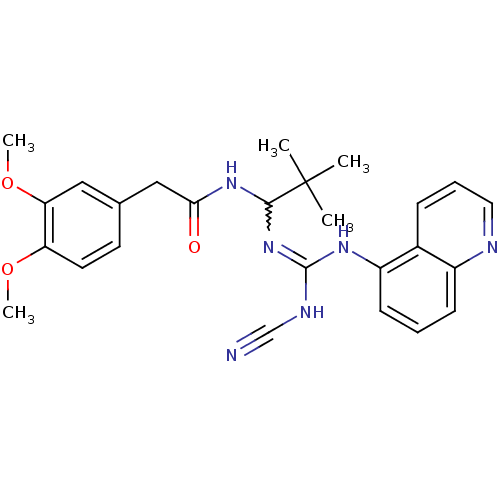
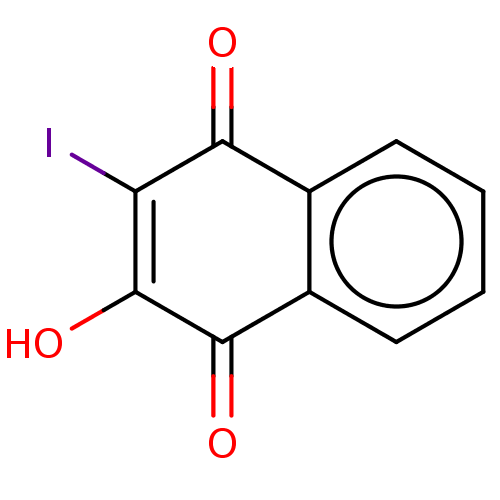
Affinity DataKi: 3.10E+3nMAssay Description:Competitive inhibition of recombinant human KLK1 expressed in baculovirus/insect cell expression system using Abz-KLRSSKQ-EDDnp peptide as substrate ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 0.331nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 0.479nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 0.490nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 0.575nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 0.794nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 0.955nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 1.29nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 2.51nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 2.82nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 2.88nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 3.63nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 3.63nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 4.27nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 4.57nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 5.13nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of ATP-induced ethidium iodide uptake preincubated for 10...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 5.75nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60nMAssay Description:Tested for inhibitory potency against rat liver microsomal squalene synthaseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 6.76nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 7.08nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 7.24nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 7.76nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 8.32nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 8.71nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 8.71nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 9.33nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 9.77nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 12.0nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 14.1nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 15.8nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 19.9nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetP2X purinoceptor 7(Rattus norvegicus (Rat))
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Tested for inhibitory potency against rat liver microsomal squalene synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced platelet aggregation in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced platelet aggregation in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Antagonist activity at human P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced ethidium iodide uptake preincubated for 10 mins fo...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 22.9nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Antagonist activity at P2X7R in Swiss Webster mouse peritoneal macrophages assessed as inhibition of ATP-induced propidium iodide uptake preincubated...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 25.1nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 25.7nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 30.2nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 33.1nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced contraction of rat isolated thoracic aortic stripMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 39.8nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Antagonist activity at P2X7 receptor in Swiss mouse peritoneal macrophages assessed as inhibition of BzATP-induced current at holding potential of -6...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
Affinity DataIC50: 42.7nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Antagonist activity at P2X7 receptor in Swiss mouse peritoneal macrophages assessed as inhibition of BzATP-induced current at holding potential of -6...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Antagonist activity at P2X7R in Swiss Webster mouse peritoneal macrophages assessed as inhibition of ATP-induced propidium iodide uptake preincubated...More data for this Ligand-Target Pair