In DepthDetails
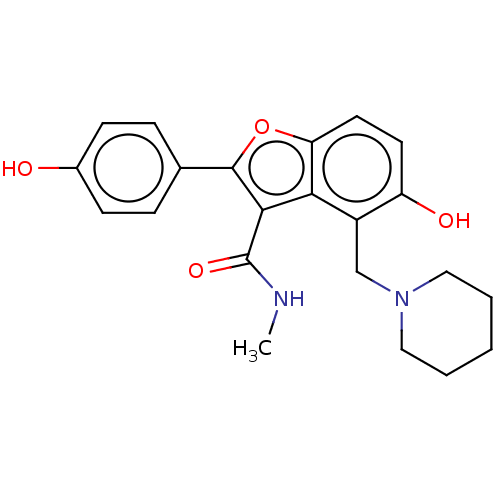
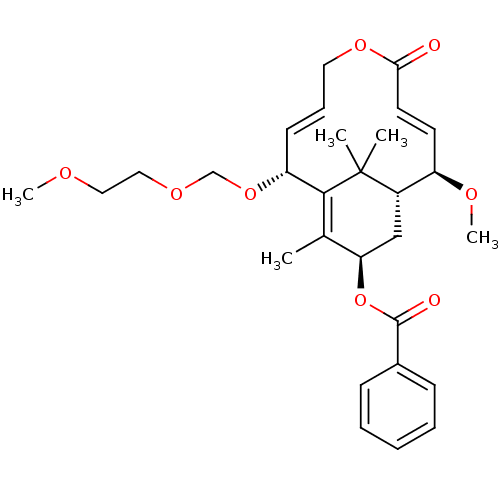
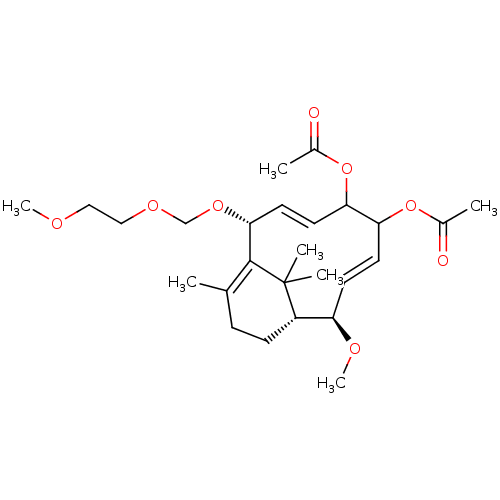
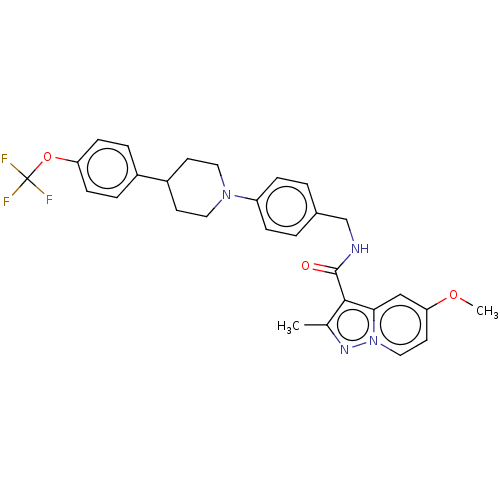
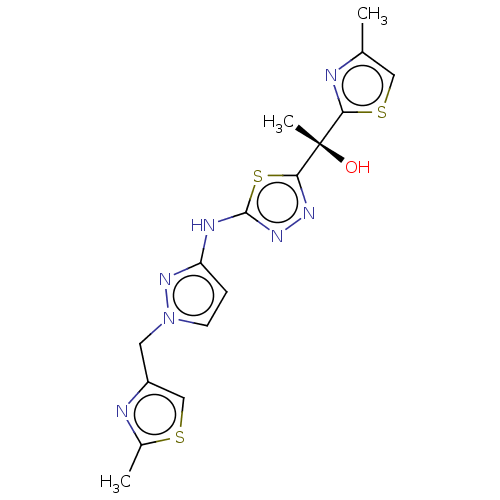
TargetDecaprenylphosphoryl-beta-D-ribose oxidase(Mycobacterium smegmatis (strain ATCC 700084 / mc(2...)TBA
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Mycobacterium tuberculosis (strain ATCC 25618 / H3...)TBA
In DepthDetails
Affinity DataIC50: 670nMAssay Description:Inhibition of human MDR1 expressed in mouse L5178 cells assessed as increase in intracellular accumulation of rhodamine 123 by FACSCalibur flow cytom...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of human MDR1 expressed in mouse L5178 cells assessed as increase in intracellular accumulation of rhodamine 123 by FACSCalibur flow cytom...More data for this Ligand-Target Pair
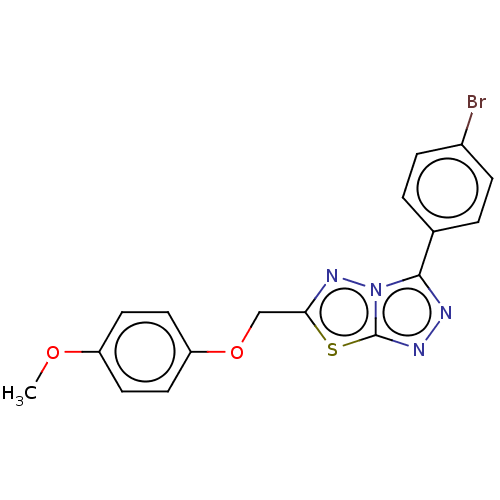
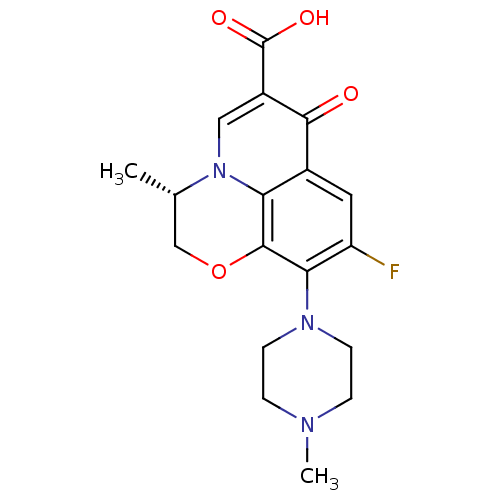
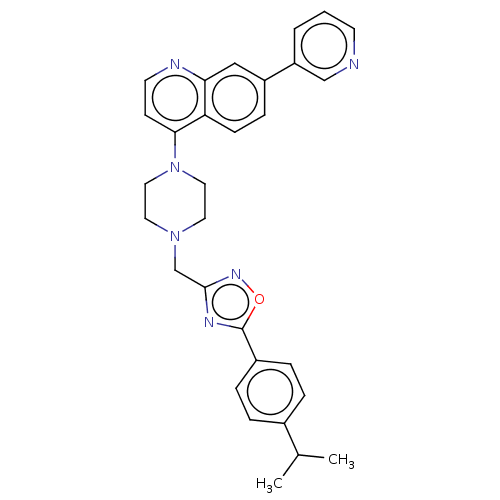
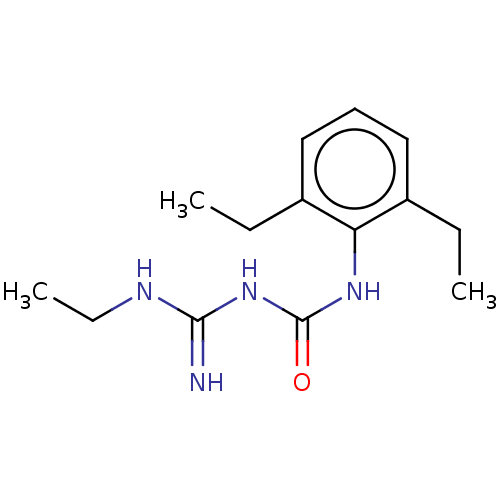
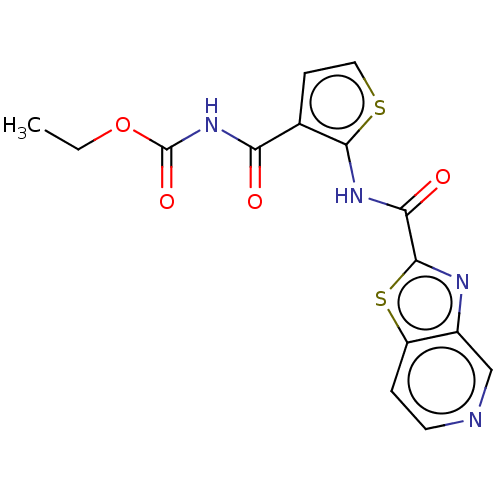
TargetDNA topoisomerase 4 subunit A/B(Staphylococcus aureus)
Sapienza University of Rome
Curated by ChEMBL
Sapienza University of Rome
Curated by ChEMBL
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of Staphylococcus aureus topoisomerase-4 expressed in Escherichia coli assessed as relaxation of pBR322 substrate measured after 4 hrs by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase S84L mutantMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of human MDR1 expressed in mouse L5178 cells assessed as increase in intracellular accumulation of rhodamine 123 by FACSCalibur flow cytom...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 4.36E+4nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase S84L mutantMore data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
In DepthDetails
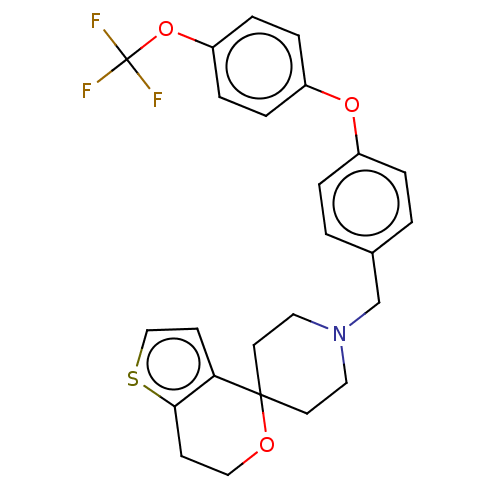
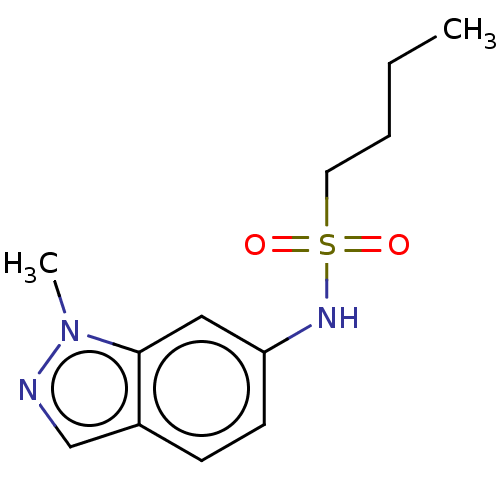
TargetDNA topoisomerase 4 subunit A/B(Staphylococcus aureus)
Sapienza University of Rome
Curated by ChEMBL
Sapienza University of Rome
Curated by ChEMBL
Affinity DataIC50: 6.16E+4nMAssay Description:Inhibition of Staphylococcus aureus topoisomerase-4 expressed in Escherichia coli assessed as relaxation of pBR322 substrate measured after 4 hrs by ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.88E+4nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase S84L mutantMore data for this Ligand-Target Pair
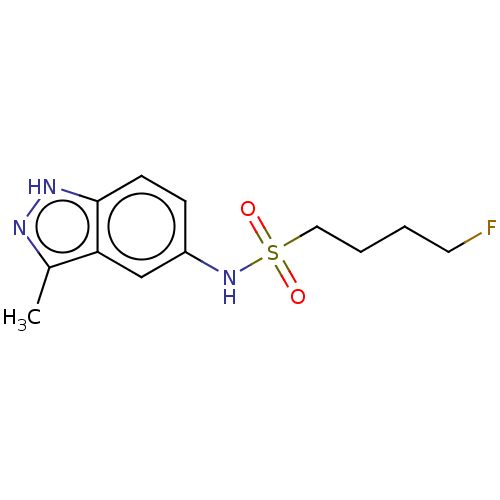
Affinity DataIC50: 5.45E+5nMAssay Description:Inhibition of Staphylococcus aureus DNA gyrase S84L mutantMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)